Choroid plexus free-water correlates with glymphatic function in Alzheimer's disease
- PMID: 40394891
- PMCID: PMC12092370
- DOI: 10.1002/alz.70239
Choroid plexus free-water correlates with glymphatic function in Alzheimer's disease
Abstract
Introduction: Free-water imaging of the choroid plexus (CP) may improve the evaluation of Alzheimer's disease (AD).
Methods: Our study investigated the role of free-water fraction (FWf) of CP in AD among 216 participants (133 Aβ+ participants and 83 Aβ- controls) enrolled in the NeuroBank-Dementia cohort at Ruijin Hospital (RJNB-D). The Alzheimer's Disease Neuroimaging Initiative dataset was used for external validation.
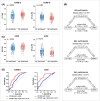
Results: At baseline, Aβ+ participants showed higher CP FWf, increased white matter hyperintensity (WMH) volume, and decreased diffusion tensor image analysis along the perivascular space (DTI-ALPS). In Aβ+ participants, DTI-ALPS mediated the association between CP FWf and periventricular WMH. CP FWf was associated with cortical tau accumulation, synaptic loss, hippocampal and cortical atrophy, and cognitive performance. During follow-up, CP FWf increased faster in Aβ+ participants than controls.
Discussion: Elevated CP FWf indicated impaired glymphatic function and AD neurodegeneration, and can be a sensitive biomarker for AD progression. The study was registered on ClinicalTrials.gov (NCT05623124).
Highlights: This cohort study found higher free-water fraction (FWf) of the choroid plexus (CP) in amyloid beta (Aβ)+ participants. CP FWf was related to glymphatic function, brain atrophy, tau burden, synaptic loss, and cognition. Aβ+ participants showed faster growth of CP FWf than Aβ- controls during follow-up. The growth rate of CP FWf exceeded that of white matter lesion and tau accumulation in Aβ+ participants. CP FWf can serve as a sensitive imaging marker of glymphatic function and Alzheimer's disease progression.
Keywords: Alzheimer's disease; choroid plexus; diffusion tensor image analysis along the perivascular space; free‐water mapping; white matter hyperintensity.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Fang Xie is an associate editor of
Figures


References
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

