The molecular mechanism of ATP synthase constrains the evolutionary landscape of chemiosmosis
- PMID: 40394897
- PMCID: PMC12256841
- DOI: 10.1016/j.bpj.2025.05.017
The molecular mechanism of ATP synthase constrains the evolutionary landscape of chemiosmosis
Abstract
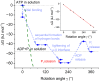
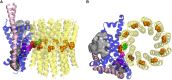
ATP synthase, the enzyme responsible for regenerating adenosine triphosphate (ATP) in the cell, comprises a proton-translocating motor in the cell membrane (labeled FO in bacteria, mitochondria, and chloroplasts), coupled by a common stalk to a catalytic motor F1 that synthesizes or hydrolyzes ATP, depending on the direction of rotation. The detailed mechanisms of FO, F1 and their coupling in ATP synthase have been elucidated through structural studies, single-molecule experiments, and molecular modeling. The outcomes of this body of work are reviewed with a particular focus on the features of the mechanism that enable the high energy efficiency and reversibility of ATP synthase. Models for the origin of chemiosmosis involve either ATP synthesis (driven by the proton gradient across the membrane) or ATP hydrolysis (for pumping protons out of the cell) as a primary function, the other function being a later development enabled by the coupled nature of the two motors. The mechanism of ATP synthase and the stringent requirements on efficiency to maintain life constrain existing models and the search for the origin of chemiosmosis.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures