[Programmed cell death in paramyxovirus infection]
- PMID: 40394914
- PMCID: PMC12176543
- DOI: 10.3724/zdxbyxb-2024-0512
[Programmed cell death in paramyxovirus infection]
Abstract
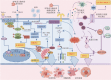
Paramyxoviruses are important respiratory pathogens with substantial clinical relevance in pediatric infectious diseases. During infection, multiple forms of programmed cell death (PCD) may be induced, and this plays pivotal roles in viral replication, dissemination, and host immune responses, thereby profoundly influencing the viral life cycle and disease progression. On one hand, PCD facilitates the clearance of infected cells, restricts viral spread, and activates host immune defenses, thereby enhancing antiviral immunity. On the other hand, excessive or dysregulated cell death may lead to tissue damage and immune imbalance, creating a microenvironment conducive to viral replication and exacerbating disease severity. For instance, apoptosis-mediated by both extrinsic and intrinsic pathways-contributes to infection control but may also be hijacked by viruses to promote dissemination. Pyroptosis, driven by inflammasome activation, triggers lytic cell death and the release of pro-inflammatory cytokines. Necroptosis, mediated by the RIPK1-RIPK3-MLKL signaling axis, and pyroptosis both amplify innate immune responses but may concurrently induce inflammatory dysregulation. Immunogenic cell death (ICD), characterized by the release of damage-associated molecular patterns and neoantigens, activates antigen-specific immune responses and holds therapeutic potential for antiviral and antitumor interventions. Emerging evidence suggests that ferroptosis, through the modulation of iron metabolism and associated transporters, may also participate in viral replication and infected cell clearance. This review comprehensively summarizes the roles of apoptosis, pyroptosis, necroptosis, ICD, and ferroptosis in paramyxovirus infection, aiming to deepen the understanding of paramyxovirus pathogenesis and to provide insights for developing novel antiviral strategies.
副黏病毒是一类重要的呼吸道病原体,在儿童感染性疾病中具有重要临床意义。其感染过程中可诱导多种形式的程序性细胞死亡(PCD),这些PCD形式在病毒复制、传播及宿主免疫应答中发挥关键作用,显著影响病毒的生命周期及疾病进展。一方面,PCD通过清除被感染细胞有助于限制病毒扩散并激活宿主免疫应答,从而增强抗病毒防御能力;另一方面,过度或异常的细胞死亡可能导致组织损伤和免疫紊乱,为病毒复制提供有利环境,进而加重病情。如细胞凋亡通过外源性与内源性通路参与感染控制,同时亦可能被病毒操控以增强其扩散能力;细胞焦亡依赖炎症小体激活,诱导裂解性死亡并释放炎症因子;坏死性凋亡通过RIPK1-RIPK3-MLKL通路介导,与细胞焦亡一样在提升先天免疫的同时可能引发炎症失衡;免疫原性细胞死亡通过释放损伤相关分子模式和新抗原激活特异性免疫反应,在抗病毒及抗肿瘤治疗中具有潜在价值;铁死亡通过调控铁代谢与相关转运蛋白参与病毒复制及细胞清除。本文综述副黏病毒感染中细胞凋亡、焦亡、坏死性凋亡、免疫原性细胞死亡及铁死亡的作用,旨在为深入理解副黏病毒的致病过程及抗病毒新策略的开发提供研究思路。.
Keywords: Immunopathology; Infectious diseases; Paramyxovirus; Pathogenesis; Programmed cell death; Review.
Conflict of interest statement
所有作者均声明不存在利益冲突. 本文由至少两名编委以外的同行专家评审,最终决定由与作者无利益冲突的其他编委作出
The authors declare that there is no conflict of interests. The manuscript was assigned to at least two independent outside reviewers, and the decision was made by other Editorial Board Members who do not have conflicts of interests with the author
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous