TE optimization for J-difference editing of 2-hydroxyglutarate at 3T
- PMID: 40395011
- PMCID: PMC12309877
- DOI: 10.1002/mrm.30561
TE optimization for J-difference editing of 2-hydroxyglutarate at 3T
Abstract
Purpose: To investigate the TE dependence of the edited 2-hydroxyglutarate (2HG) signal, its separation from co-edited glutamate plus glutamine (Glx), and fit accuracy in the presence of nuisance signals using a MEGA-PRESS sequence.
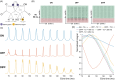
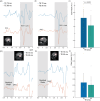
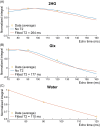
Methods: Simulations were performed at TEs 70-160 ms to assess the signal intensity and 2HG-Glx overlap as a function of TE. The effect of the 2HG-Glx spectral overlap on the fit accuracy of 2HG was evaluated on simulated 2HG-edited spectra with in vivo parameter variations. Data were acquired at TEs of 70 and 90 ms in 13 glioma patients to estimate the TE-dependence of the 2HG and Glx signal intensity and at a TE of 120 ms in eight glioma patients to estimate the in vivo 2HG, Glx, and water T2 relaxation times.
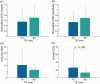
Results: A TE of 90 ms was found to produce a maximal 2HG integral, which was 23% larger than that at a TE of 70 ms in vivo without a significant increase in 2HG-Glx overlap. Lipid and residual water were 26% and 16% lower, respectively, at a TE of 90 ms versus 70 ms. Fit-quality numbers were 49% lower at a TE of 90 ms versus 70 ms, indicating enhanced fits at a TE of 90 ms. The in vivo T2 relaxation times of 2HG, Glx, and water were 264, 177, and 110 ms, respectively.
Conclusion: A TE of 90 ms was best with a maximal 2HG signal, minimal 2HG-Glx overlap, and minimal residual water and lipid contamination.
Keywords: 2HG; J‐difference editing; MR spectroscopy; glioma; tumor.
© 2025 The Author(s). Magnetic Resonance in Medicine published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

