Intraputaminal Delivery of Adeno-Associated Virus Serotype 2-Glial Cell Line-Derived Neurotrophic Factor in Mild or Moderate Parkinson's Disease
- PMID: 40395017
- PMCID: PMC12273615
- DOI: 10.1002/mds.30193
Intraputaminal Delivery of Adeno-Associated Virus Serotype 2-Glial Cell Line-Derived Neurotrophic Factor in Mild or Moderate Parkinson's Disease
Abstract
Background: Glial cell line-derived neurotrophic factor (GDNF) is required for development and survival of dopaminergic neurons. A previous trial evaluating lower-dose adeno-associated virus serotype 2-GDNF (AAV2-GDNF) bilateral intraputaminal infusion in participants with advanced Parkinson's disease (PD) achieved 26% mean putaminal coverage and was associated with stable motor features with no unexpected adverse events (AEs) over 60 months.
Objective: We assessed safety and preliminary clinical outcomes of optimized bilateral intraputaminal infusion of a single, higher dose of AAV2-GDNF (product code AB-1005) for PD after 18 months.
Methods: This phase 1b single-arm, open-label clinical trial enrolled participants with mild (Movement Disorder Society-revised Unified Parkinson's Disease Rating Scale [MDS-UPDRS] Part III off score ≤ 32) and moderate (MDS-UPDRS Part III off score of 33-60) PD. The primary outcome was safety. Clinical outcomes were assessed using PD-specific clinical measures.
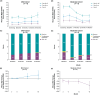
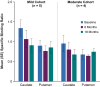
Results: Eleven participants were enrolled (n = 6 mild; n = 5 moderate). Mean (±SE) putaminal coverage of AAV2-GDNF was 63% (±2%). All participants experienced treatment-emergent AEs (63 events); most were transient and perioperative. Six serious AEs in three participants were unrelated to AAV2-GDNF. At 18 months posttreatment, the mild cohort exhibited numerically stable MDS-UPDRS, motor diary, Unified Dyskinesia Rating Scale (UDysRS) scores, and levodopa equivalent daily dose (LEDD). The moderate cohort demonstrated numerical improvements in mean (±SE) MDS-UPDRS Part III off scores (-20.4 [±4.5]), motor diary off time (-1.7 [±1.1] hours), and UDysRS scores (-2.2 [±1.9]) and reduced LEDD (-257.6 [±162.2] mg).
Conclusions: Bilateral intraputaminal AAV2-GDNF gene therapy was well tolerated and associated with numerical stability (mild cohort) and improvement (moderate cohort) in clinical assessments at 18 months posttreatment. © 2025 AskBio Inc and The Author(s). Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Keywords: GDNF; Parkinson's Disease; gene therapy; movement disorders.
© 2025 AskBio Inc and The Author(s). Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Figures

References
-
- Behl T, Kaur I, Kumar A, Mehta V, Zengin G, Arora S. Gene therapy in the management of Parkinson's disease: potential of GDNF as a promising therapeutic strategy. Curr Gene Ther 2020;20(3):207–222. - PubMed
-
- Kramer ER, Liss B. GDNF‐ret signaling in midbrain dopaminergic neurons and its implication for Parkinson disease. FEBS Lett 2015;589(24 Pt A):3760–3772. - PubMed
-
- Pascual A, Hidalgo‐Figueroa M, Piruat JI, Pintado CO, Gomez‐Diaz R, Lopez‐Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci 2008;11(7):755–761. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

