Increased absorptive transcytosis and tight junction weakness in heart failure are equally corrected by exercise training and losartan
- PMID: 40395026
- PMCID: PMC12203988
- DOI: 10.1042/CS20242965
Increased absorptive transcytosis and tight junction weakness in heart failure are equally corrected by exercise training and losartan
Abstract
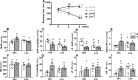
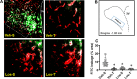
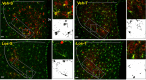
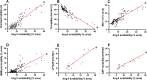
Reduced ventricular function, renin-angiotensin system upregulation and sympathoexcitation are hallmarks of heart failure (HF). Recently we showed that blood-brain barrier (BBB) lesion within autonomic nuclei contributes to autonomic imbalance and that exercise training (T) normalizes BBB function and improves autonomic control. We sought now to identify the mechanism( involved in both HF-induced lesion and exercise-induced correction. Wistar rats submitted to coronary artery ligation were, after the development of HF, assigned to losartan (Los) or vehicle (Veh) treatments and simultaneously submitted to T or sedentary ( protocol. After hemodynamic/autonomic recordings and evaluation of BBB permeability, brains were harvested for ultrastructural analyses of the barrier (tight junctions (TJ) tightness and vesicles trafficking) within capillaries of the hypothalamic paraventricular nucleus. Local angiotensin II (Ang II) expression and activation of microglial cells (IBA-1 immunofluorescence) were also evaluated. High sympathetic activity and pressure variability, reduced parasympathetic control of the heart, elevated BBB permeability, high vesicular trafficking and TJ weakness exhibited by Veh-rats were equally corrected in Veh-T, Los-and Los-T groups. The increased PVN Ang II expression and IBA-1 density in Veh-group were similarly reduced by T, Los and combination of both. Ang II, colocalized with microglia AT1 receptors, induced their remodeling from disease-associated phenotype in Veh-S rats to homeostatic-surveilling conditions in the other groups. All measured parameters exhibited strong correlations with Ang II availability. Data indicated that changes in PVN Ang II availability induced by HF, exercise and losartan is the key regulator of transcellular and paracellular transport across the BBB.
Keywords: absorptive transcytosis; angiotensin II; blood-brain barrier; heart failure; microglia; tight junctions.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures





Similar articles
-
Blood-brain barrier lesion - a novel determinant of autonomic imbalance in heart failure and the effects of exercise training.Clin Sci (Lond). 2023 Aug 14;137(15):1049-1066. doi: 10.1042/CS20230489. Clin Sci (Lond). 2023. PMID: 37477956
-
Hypertension-induced heart failure disrupts cardiac sympathetic innervation.Am J Physiol Heart Circ Physiol. 2024 Dec 1;327(6):H1544-H1558. doi: 10.1152/ajpheart.00380.2024. Epub 2024 Nov 1. Am J Physiol Heart Circ Physiol. 2024. PMID: 39485300
-
Trained hypertensive rats exhibit decreased transcellular vesicle trafficking, increased tight junctions' density, restored blood-brain barrier permeability and normalized autonomic control of the circulation.Front Physiol. 2023 Feb 23;14:1069485. doi: 10.3389/fphys.2023.1069485. eCollection 2023. Front Physiol. 2023. PMID: 36909225 Free PMC article.
-
Physical exercise training interventions for children and young adults during and after treatment for childhood cancer.Cochrane Database Syst Rev. 2016 Mar 31;3(3):CD008796. doi: 10.1002/14651858.CD008796.pub3. Cochrane Database Syst Rev. 2016. PMID: 27030386 Free PMC article.
-
A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs.Epilepsia. 2012 Feb;53(2):272-82. doi: 10.1111/j.1528-1167.2011.03361.x. Epub 2012 Jan 5. Epilepsia. 2012. PMID: 22221253
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

