Engineering Bacterial Secretion Systems for Enhanced Tumor Imaging and Surgical Guidance
- PMID: 40395153
- PMCID: PMC12392856
- DOI: 10.1002/adma.202504389
Engineering Bacterial Secretion Systems for Enhanced Tumor Imaging and Surgical Guidance
Abstract
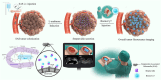
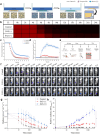
Current imaging techniques suffer from a lack of specificity and resolution, leading to inaccurate tumor imaging and limited applicability of targeted contrast agents, as they require cancer-specific development. The need for enhanced contrast through improved tumor-to-background ratio (TBR) and the toxicity from repeated injections due to fading fluorescent signals further complicate the issue. Additionally, challenges in visualizing the entire 3D tumor with surface-stained contrast agents highlight the demand for advanced imaging solutions for more precise surgical guidance. A novel approach is proposed utilizing Streptavidin Associated Salmonella (SAS) as a contrast agent for image-guided surgeries. SAS selectively proliferates in cancerous tissues and secretes streptavidin upon induction, enabling the binding of subsequently injected biotin-conjugated fluorescent dyes. This approach enhances tumor visualization with a TBR of up to 15.3, far surpassing conventional agents (TBR ∼ 2), while enabling prolonged 3-day imaging, deep tumor penetration, and precise invasive margin delineation with a single contrast agent injection. Furthermore, biosafety evaluations confirmed efficient bacterial clearance, absence of systemic toxicity, and stable physiological responses, supporting its potential for safe clinical translation. This innovative method offers substantial improvements over existing fluorescent contrast agents and holds promise for both diagnostic and therapeutic applications in cancer surgery.
Keywords: bacteria‐based tumor visualization; fluorescence image‐guided surgery; fluorescent contrast agents; streptavidin‐associated Salmonella (SAS); surgical guidance.
© 2025 The Author(s). Advanced Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Lyon F., global Cancer Burden Growing, Amidst Mounting Need For Services, https://www.who.int/news/item/01‐02‐2024‐global‐cancer‐burden‐growing–am..., “[Online; World Health Organization News]” 2024. - PMC - PubMed
-
- Orosco R. K., Tapia V. J., Califano J. A., Clary B., Cohen E. E., Kane C., Lippman S. M., Messer K., Molinolo A., Murphy J. D., Tapia V. J., Califano J. A., Clary B., Cohen E. E. W., Kane C., Lippman S. M., Messer K., Molinolo A., Murphy J. D., Pang J., Sacco A., Tringale K. R., Wallace A., Nguyen Q. T., Sci. Rep. 2018, 8, 5686. - PMC - PubMed
-
- Mieog J. S. D., Achterberg F. B., Zlitni A., Hutteman M., Burggraaf J., Swijnenburg R.‐J., Gioux S., Vahrmeijer A. L., Nat. Rev. Clin. Oncol. 2022, 19, 9. - PubMed
-
- Wang K., Chi C., Hu Z., Liu M., Hui H., Shang W., Peng D., Zhang S., Ye J., Liu H., et al., Engineering 2015, 1, 309.
-
- Stewart H. L., Birch D. J., Methods and Applications in Fluorescence 2021, 9, 042002. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

