COP1 drives renal cell carcinoma progression by targeting ACSL4 for ubiquitin-mediated degradation and inhibiting ferroptosis
- PMID: 40395328
- PMCID: PMC12089050
- DOI: 10.3389/fonc.2025.1570727
COP1 drives renal cell carcinoma progression by targeting ACSL4 for ubiquitin-mediated degradation and inhibiting ferroptosis
Abstract
Background: Renal cell carcinoma (RCC) progression is closely linked to dysregulation of the ubiquitin-proteasome system, particularly aberrant ubiquitination processes governing protein degradation and cell cycle control. As a pivotal E3 ubiquitin ligase, COP1 mediates substrate-specific ubiquitination to regulate protein stability. However, its functional role in RCC remains poorly characterized. This study investigates how COP1 drives RCC malignancy and explores its underlying molecular mechanisms.
Methods: We analyzed the expression of COP1 in RCC cells and its relationship with patient overall survival (OS) in databases. The CCK-8 assay was used to detect the effect of COP1 on the proliferation of RCC cells, while the Transwell assay was used to assess the impact of COP1 on the migration and invasion of RCC cells. We employed mass spectrometry, co-immunoprecipitation, Western blot, and RT-qPCR to explore the target proteins that interact with COP1 and their interaction modes. After inducing with ferroptosis inducers, we measured the effect of COP1 on lipid ROS levels in RCC cells. Finally, we validated the role of COP1 in RCC using in vivo experiments.
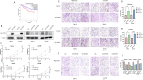
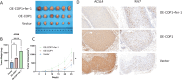
Results: COP1 was significantly correlated with poor patient prognosis. Functional studies demonstrated that COP1 overexpression markedly increased RCC cell proliferation by 65% (786-O) and 58% (ACHN) (p < 0.001) and enhanced migration/invasion (p < 0.01), while COP1 knockdown suppressed these malignant phenotypes by 40-50%. Mechanistically, COP1 directly bound ACSL4 and promoted its K48-linked ubiquitination, reducing ACSL4 protein stability by 70% (p < 0.001) and suppressing ferroptosis, as evidenced by decreased lipid ROS levels (p < 0.01) and reversal of ferroptosis inhibition by ferrostatin-1. In vivo, COP1 overexpression accelerated tumor growth in xenograft models, with a 2.5-fold increase in tumor volume compared to controls (p < 0.001), accompanied by reduced ACSL4 expression and elevated Ki67 proliferation index. These effects were further amplified by the ferroptosis inhibitor ferrostatin-1, underscoring COP1's role in driving tumor progression through ferroptosis suppression.
Conclusion: Our study establishes COP1 as a critical driver of RCC progression by suppressing ferroptosis through ubiquitin-mediated degradation of ACSL4, thereby providing a novel theoretical foundation for targeted therapeutic strategies in RCC.
Keywords: ACSL4; COP1; RCC; ferroptosis; ubiquitination.
Copyright © 2025 Zheng, Jiang, Qi and Peng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Tyrosine phosphatase SHP2 promoted the progression of CRC via modulating the PI3K/BRD4/TFEB signaling induced ferroptosis.Discov Oncol. 2024 Dec 18;15(1):793. doi: 10.1007/s12672-024-01586-w. Discov Oncol. 2024. PMID: 39692787 Free PMC article.
-
SENP1 inhibits ferroptosis and promotes head and neck squamous cell carcinoma by regulating ACSL4 protein stability via SUMO1.Oncol Rep. 2024 Feb;51(2):34. doi: 10.3892/or.2023.8693. Epub 2024 Jan 8. Oncol Rep. 2024. PMID: 38186303 Free PMC article.
-
COP1 is downregulated in renal cell carcinoma (RCC) and inhibits the migration of RCC ACHN cells in vitro.Mol Med Rep. 2016 Aug;14(2):1371-8. doi: 10.3892/mmr.2016.5373. Epub 2016 Jun 7. Mol Med Rep. 2016. PMID: 27278120
-
The mechanism of E3 ubiquitin ligase HERC1 regulating ferroptosis in lung adenocarcinoma cells.Cancer Genet. 2025 Apr;292-293:92-99. doi: 10.1016/j.cancergen.2025.02.001. Epub 2025 Feb 3. Cancer Genet. 2025. PMID: 39983667
-
CLIP170 enhancing FOSL1 expression via attenuating ubiquitin-mediated degradation of β-catenin drives renal cell carcinoma progression.Cell Mol Life Sci. 2024 Nov 28;81(1):467. doi: 10.1007/s00018-024-05504-9. Cell Mol Life Sci. 2024. PMID: 39607512 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

