Computational drug discovery of potential 5α-reductase phytochemical inhibitors and hair growth promotion using in silico techniques
- PMID: 40395369
- PMCID: PMC12089051
- DOI: 10.3389/fbinf.2025.1570101
Computational drug discovery of potential 5α-reductase phytochemical inhibitors and hair growth promotion using in silico techniques
Abstract
Introduction: Male pattern hair loss (MPHL), also known as androgenetic alopecia (AGA), is a common disorder primarily caused by dihydrotestosterone (DHT). The Food and Drug Administration (FDA) has approved two 5-alpha reductase (5-AR) inhibitors-finasteride and dutasteride-for treating this condition. However, recent studies have reported adverse sexual side effects and issues with sperm production in young men using these medications. There are also recommendations for effectively treating hair loss with natural remedies, such as Urtica dioica (nettle), Serenoa repens (saw palmetto), and Trigonella foenum-graecum (fenugreek) that is mainly used for diminish the hair loss in the traditional medicine. Research shows that these herbal formulations and plant extracts may help reduce hair loss. However, the concentration of active compounds in these herbal extracts is often low, necessitating a large extract volume to achieve noticeable effects on hair growth. Although many studies have investigated the effects of these herbal extracts on hair growth, fewer studies focus on the specific compounds influencing the molecular mechanisms of hair loss, particularly the inhibition of 5-AR.
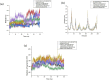
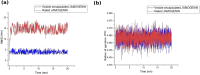
Methods: For the first time, we aimed to applied a computational study to explore the phytochemicals extracted from these herbs to identify compounds that can effectively bind to and inhibit 5-AR. Additionally, we assessed the stability of the ligands encapsulated in lipid nanoparticles (LNP) by conducting molecular dynamics (MD) simulations of the LNP-encapsulated ligands. We utilized an online database to identify compounds from the extracts of nettle, saw palmetto, and fenugreek. We then analyzed their binding affinity to 5-AR using computational techniques.
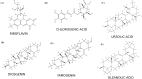
Results: We found that 6 molecules-Jamogenin, Neodiosgenin, Chlorogenic acid, Rutin, Riboflavin, and Ursolic acid-are effective in binding to 5-AR. Additionally, our in silico studies revealed that vesicle-entrapped JAMOGENIN, which has a stronger bond with 5-AR, is more stable than its unencapsulated form.
Discussion: Therefore, these 6 molecules, particularly JAMOGENIN, should be considered for experimental analysis in both their unencapsulated and nanocarrier-encapsulated states to promote hair follicle growth.
Keywords: 5α-reductase inhibitors; androgenetic alopecia; computational study; hair follicle growth; herbal extracts.
Copyright © 2025 Hasannejad-Asl, Pooresmaeil, azadi, Najafi, Esmaeili, Bagheri-Mohammadi and Kazemi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
An overview of herbal alternatives in androgenetic alopecia.J Cosmet Dermatol. 2019 Aug;18(4):966-975. doi: 10.1111/jocd.12930. Epub 2019 Apr 13. J Cosmet Dermatol. 2019. PMID: 30980598 Review.
-
Evaluation of the therapeutic effects of AGA drugs by measuring finasteride, dutasteride, and dihydrotestosterone in hair.Clin Chim Acta. 2023 Jul 1;547:117456. doi: 10.1016/j.cca.2023.117456. Epub 2023 Jun 28. Clin Chim Acta. 2023. PMID: 37385468
-
Comparison of oral minoxidil, finasteride, and dutasteride for treating androgenetic alopecia.J Dermatolog Treat. 2022 Nov;33(7):2946-2962. doi: 10.1080/09546634.2022.2109567. Epub 2022 Aug 15. J Dermatolog Treat. 2022. PMID: 35920739 Review.
-
In silico Analysis of Berberine as a Potential Therapeutic Approach for Various Signalling Pathways Linked to Androgenetic Alopecia.Recent Adv Inflamm Allergy Drug Discov. 2025;19(1):71-78. doi: 10.2174/0127722708307894240624105514. Recent Adv Inflamm Allergy Drug Discov. 2025. PMID: 40195703
-
Natural Compounds Used for Treating Hair Loss.Curr Pharm Des. 2023;29(16):1231-1244. doi: 10.2174/1381612829666230505100147. Curr Pharm Des. 2023. PMID: 37151166 Review.
Cited by
-
A SARM a Day Keeps the Weakness Away: A Computational Approach for Selective Androgen Receptor Modulators (SARMs) and Their Interactions with Androgen Receptor and 5‑Alpha Reductase Proteins.ACS Omega. 2025 Jul 18;10(29):31649-31667. doi: 10.1021/acsomega.5c02504. eCollection 2025 Jul 29. ACS Omega. 2025. PMID: 40757306 Free PMC article.
References
-
- Abdurrahman S., Ruslin R., Hasanah A. N., Ifaya M., Mustarichie R. (2023). Anti-alopecia activity of coumarin derivatives isolated from Merremia peltata leaves and computational study of their binding to androgen receptors using molecular docking and molecular dynamic simulation. Pharmaceuticals 16 (5), 669. 10.3390/ph16050669 - DOI - PMC - PubMed
-
- Abraham M., Alekseenko A., Bergh C., Blau C., Briand E., Doijade M., et al. (2023). GROMACS 2023.2 manual.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

