Activation of perfluoro(methyl vinyl ether) at Rh(i) complexes: metal-centered versus phosphine-mediated decarbonylation
- PMID: 40395377
- PMCID: PMC12086712
- DOI: 10.1039/d5sc02056e
Activation of perfluoro(methyl vinyl ether) at Rh(i) complexes: metal-centered versus phosphine-mediated decarbonylation
Abstract
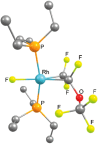
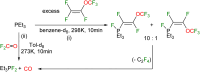
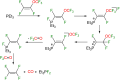
This study investigates the reactivity of perfluoro(methyl vinyl ether) [PMVE, CF2[double bond, length as m-dash]CF(OCF3)] towards rhodium(i) phosphine complexes. The reaction pathways are characterized by C-O and C-F bond cleavage reactions and decarbonylation steps. On using the complex [Rh(H)(PEt3)3] (1), unprecedented reactivity pathways were observed that are distinct from those found for previously studied fluoroolefins. Reactivity of an excess PMVE at Rh is initiated by coordination to the Rh center in 1, followed by its insertion into the Rh-H bond and a β-OCF3 elimination. This process ultimately results in OCF3 ligand transformation to give trans-[Rh(F)(CO)(PEt3)3] (4) and Et3PF2. Reactions of stoichiometric amounts of PMVE with [Rh(H)(PEt3)3] (1) or an excess amount of it with [Rh(F)(PEt3)3] (6) led to olefin complex formation to yield trans-[Rh(F)(η2-CF2CFH)(PEt3)2] (7) and trans-[Rh(F)(CF(OCF3)CF2)(PEt3)2] (8), respectively. In contrast, a remarkable insertion into the Rh-F bond at [Rh(F)(CO)(PEt3)2] (4) was observed leading to the formation of trans-[Rh(CO)(CF(OCF3)CF3)(PEt3)2] (5). Decarbonylation of PMVE proceeds not only at Rh, but also via a metal-free, phosphine-mediated process. The latter is characterized by oxidative addition of PMVE at PEt3 to form the fluorophosphoranes E/Z-(F3CO)CF[double bond, length as m-dash]CF(PFEt3), which subsequently convert into Et3PF2, CO and presumably tetrafluoroethene.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




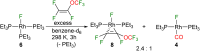
Similar articles
-
Organoarsine Metal-Organic Framework as a Solid-State Ligand for Rhodium(I) Olefin Hydroformylation Catalysis.J Am Chem Soc. 2025 Aug 13;147(32):29119-29129. doi: 10.1021/jacs.5c07706. Epub 2025 Jul 29. J Am Chem Soc. 2025. PMID: 40729213
-
Versatile Reaction Pathways of 1,1,3,3,3-Pentafluoropropene at Rh(I) Complexes [Rh(E)(PEt3 )3 ] (E=H, GePh3 , Si(OEt)3 , F, Cl): C-F versus C-H Bond Activation Steps.Chemistry. 2021 Aug 16;27(46):11926-11934. doi: 10.1002/chem.202101508. Epub 2021 Jun 28. Chemistry. 2021. PMID: 34118095 Free PMC article.
-
Synthesis of a rhodium(i) germyl complex: a useful tool for C-H and C-F bond activation reactions.Dalton Trans. 2016 Mar 21;45(11):4716-28. doi: 10.1039/c5dt04845a. Epub 2016 Feb 10. Dalton Trans. 2016. PMID: 26863494
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Prevalence and odds of anxiety and depression in cutaneous malignant melanoma: a proportional meta-analysis and regression.Br J Dermatol. 2024 Jun 20;191(1):24-35. doi: 10.1093/bjd/ljae011. Br J Dermatol. 2024. PMID: 38197404
References
-
- Heath E. A. Int. Leg. Mater. 2017;56:193–205. doi: 10.1017/ilm.2016.2. - DOI
-
- Manahan S. E., Fundamentals of environmental and toxicological chemistry: sustainable science, CRC press, 2013
-
- Kirsch P., Modern Fluoroorganic Chemistry: Synthesis, Reactivity and Applications, Wiley-VCH, Weinheim, 2nd edn, 2013
LinkOut - more resources
Full Text Sources
Miscellaneous

