Methods for detection of cardiac glycogen-autophagy
- PMID: 40395528
- PMCID: PMC11864643
- DOI: 10.1080/27694127.2024.2405331
Methods for detection of cardiac glycogen-autophagy
Abstract
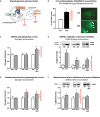
Glycogen-autophagy ('glycophagy') is a selective autophagy process involved in delivering glycogen to the lysosome for bulk degradation. Glycophagy protein intermediaries include STBD1 as a glycogen tagging receptor, delivering the glycogen cargo into the forming phagosome by partnering with the Atg8 homolog, GABARAPL1. Glycophagy is emerging as a key process of energy metabolism and development of reliable tools for assessment of glycophagy activity is an important priority. Here we show that antibodies raised against the N-terminus of the GABARAPL1 protein (but not the full-length protein) detected a specific endogenous GABARAPL1 immunoblot band at 18kDa. A stable GFP-GABARAPL1 cardiac cell line was used to quantify GABARAPL1 lysosomal flux via measurement of GFP puncta in response to lysosomal inhibition with bafilomycin. Endogenous glycophagy flux was quantified in primary rat ventricular myocytes by the extent of glycogen accumulation with bafilomycin combined with chloroquine treatment (no effect observed with bafilomycin or chloroquine alone). In wild-type isolated mouse hearts, bafilomycin alone and bafilomycin combined with chloroquine (but not chloroquine alone) elicited a significant increase in glycogen content signifying basal glycophagy flux. Collectively, these methodologies provide a comprehensive toolbox for tracking cardiac glycophagy activity to advance research into the role of glycophagy in health and disease.
Keywords: Atg8; GABARAPL1; autophagy; glycogen; glycophagy flux.
© 2024 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Update of
-
Methods for detection of cardiac glycogen-autophagy.bioRxiv [Preprint]. 2024 Aug 11:2024.08.11.607511. doi: 10.1101/2024.08.11.607511. bioRxiv. 2024. Update in: Autophagy Rep. 2024 Sep 22;3(1):2405331. doi: 10.1080/27694127.2024.2405331. PMID: 39211188 Free PMC article. Updated. Preprint.
References
-
- Johansen T, Lamark T.. Selective Autophagy: ATG8 Family Proteins, LIR Motifs and Cargo Receptors. J Mol Biol 2020; 432:80–103. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
