The Pregnane-X receptor regulates steroid synthesis in mouse Leydig cells
- PMID: 40395611
- PMCID: PMC12088964
- DOI: 10.3389/fendo.2024.1430781
The Pregnane-X receptor regulates steroid synthesis in mouse Leydig cells
Abstract
Introduction: Pregnane X Receptor (PXR, NR1I2) is a ligand-dependent transcription factor belonging to the nuclear receptor superfamily, that can be activated by a wide variety of endogenous and exogenous ligands. It is a major actor of the endo- and xeno-biotic detoxification process. It also regulates biological processes such as lipid metabolism in large number of tissues. Pxr was shown to be expressed in human, mouse, rat and pig testis, however its roles in the regulation of testicular functions have been little explored so far.
Methods: To determine the potential involvement of PXR in the regulation of steroidogenesis, experiments were performed on a wild type (MLTC-1WT) and a Pxr knock-down (MLTC-1PxrKD) mouse Leydig cell line (MLTC-1 cells), treated with a PXR agonist (SR-12813) in acute and chronic conditions.
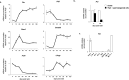
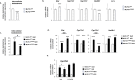
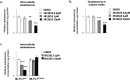
Results: Our analyses confirmed the presence of Pxr transcripts in the mouse testis, particularly in Leydig cells. In addition, A lower testosterone concentration was measured in MLTC-1PxrKD cells compared to wild type cells. Moreover, both acute and chronic stimulation of MLTC-1WT cells with SR-12813 led to a decrease in testosterone concentration, associated with a lower expression of some steroidogenic genes. This negative impact of SR-12813 on Leydig cell steroidogenesis was counteracted by Pxr knock down.
Discussion: Overall, these results support the involvement of PXR in the regulation of testosterone homeostasis in mouse Leydig cells and open new avenues of research into the involvement of this receptor in the deleterious effects of certain endocrine disruptors on the steroidogenic activity of Leydig cells.
Keywords: Leydig cells; PXR; mouse; testosterone; xenobiotics.
Copyright © 2025 Martinot, Holota, de Haze, Beaudoin and Volle.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
The hepatokine FGF21 stopped lipogenesis and reduced testosterone production in mLTC-1 Leydig Cell Line.Mol Cell Endocrinol. 2024 Dec 1;594:112350. doi: 10.1016/j.mce.2024.112350. Epub 2024 Sep 2. Mol Cell Endocrinol. 2024. PMID: 39233040
-
Pregnenolone 16-Alpha Carbonitrile, an Agonist of Rodent Pregnane X Receptor, Regulates Testosterone Biosynthesis in Rodent Leydig Cells.J Xenobiot. 2024 Sep 16;14(3):1256-1267. doi: 10.3390/jox14030071. J Xenobiot. 2024. PMID: 39311150 Free PMC article.
-
Cisatracurium stimulates testosterone synthesis in rat and mouse Leydig cells via nicotinic acetylcholine receptor.J Cell Mol Med. 2020 Dec;24(24):14184-14194. doi: 10.1111/jcmm.16029. Epub 2020 Oct 27. J Cell Mol Med. 2020. PMID: 33111502 Free PMC article.
-
The role of pregnane X receptor (PXR) in substance metabolism.Front Endocrinol (Lausanne). 2022 Aug 16;13:959902. doi: 10.3389/fendo.2022.959902. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36111293 Free PMC article. Review.
-
[Role of pregnane X receptor (PXR) in endobiotic metabolism].Sheng Li Xue Bao. 2019 Apr 25;71(2):311-318. Sheng Li Xue Bao. 2019. PMID: 31008491 Review. Chinese.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

