Effectiveness of Iptacopan Versus C5 Inhibitors in Complement Inhibitor-Naive Patients With Paroxysmal Nocturnal Haemoglobinuria
- PMID: 40395624
- PMCID: PMC12090361
- DOI: 10.1002/jha2.70055
Effectiveness of Iptacopan Versus C5 Inhibitors in Complement Inhibitor-Naive Patients With Paroxysmal Nocturnal Haemoglobinuria
Abstract
Background: Paroxysmal nocturnal haemoglobinuria (PNH) is characterised by haemolytic anaemia, bone marrow failure and thrombosis. The single-arm phase 3 APPOINT-PNH trial (NCT04820530) investigating iptacopan monotherapy in complement inhibitor-naive patients demonstrated significant haemoglobin concentration improvements.
Methods: We used target trial emulation to retrospectively predict outcomes if APPOINT-PNH trial patients had received C5 inhibitors instead of iptacopan. Estimates were derived from the real-world APPEX cohort treated with routine C5 inhibitors. The study used benchmarking and comparative effectiveness to evaluate the haematological response in APPOINT-PNH if patients had received C5 inhibitors. Treatment effect was estimated using propensity scores to model the probability of trial inclusion based on baseline covariates, followed by fitting an outcome model to the APPEX cohort.
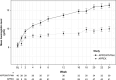
Results: The analysis of 125 patients showed all estimated treatment effects (95% confidence interval) favoured iptacopan over C5 inhibitors: differences in the proportion of patients achieving haemoglobin increase from baseline of ≥ 2 g/dL, 68.2% (40.9-95.6); haemoglobin levels of ≥ 12 g/dL, 53.4% (31.4-75.3); transfusion independence, 38.8% (15.1-62.5); ratio of percent change from baseline in lactate dehydrogenase levels, 0.51 (0.40-0.67); change from baseline in reticulocytes, -75.5 × 109/L (-106.9, -44.2).
Conclusions: Results indicate C5 inhibitor-naive patients with PNH may experience greater haematological response with iptacopan than with C5 inhibitors.
Trial registration: ClinicalTrials.gov identifier: NCT05842486.
Keywords: APPEX; APPOINT‐PNH; C5 inhibitors; iptacopan; paroxysmal nocturnal haemoglobinuria.
© 2025 The Author(s). eJHaem published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest
Figures


References
-
- Brodsky R. A., “Narrative Review: Paroxysmal Nocturnal Hemoglobinuria: The Physiology of Complement‐Related Hemolytic Anemia,” Annals of Internal Medicine 148, no. 8 (2008): 587–595. - PubMed
-
- Richards S. J., Painter D., Dickinson A. J., et al., “The Incidence and Prevalence of Patients With Paroxysmal Nocturnal Haemoglobinuria and Aplastic Anaemia PNH Syndrome: A Retrospective Analysis of the UK's Population‐Based Haematological Malignancy Research Network 2004–2018,” European Journal of Haematology 107, no. 2 (2021): 211–218. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
