Patient-derived tumor models and their distinctive applications in personalized drug therapy
- PMID: 40395637
- PMCID: PMC12082161
- DOI: 10.1016/j.mbm.2023.100014
Patient-derived tumor models and their distinctive applications in personalized drug therapy
Abstract
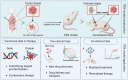
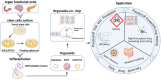
Tumor models in vitro are conventional methods for developing anti-cancer drugs, evaluating drug delivery, or calculating drug efficacy. However, traditional cell line-derived tumor models are unable to capture the tumor heterogeneity in patients or mimic the interaction between tumors and their surroundings. Recently emerging patient-derived preclinical cancer models, including of patient-derived xenograft (PDX) model, circulating tumor cell (CTC)-derived model, and tumor organoids-on-chips, are promising in personalized drug therapy by recapitulating the complexities and personalities of tumors and surroundings. These patient-derived models have demonstrated potential advantages in satisfying the rigorous demands of specificity, accuracy, and efficiency necessary for personalized drug therapy. However, the selection of suitable models is depending on the specific therapeutic requirements dictated by cancer types, progressions, or the assay scale. As an example, PDX models show remarkable advantages to reconstruct solid tumors in vitro to understand drug delivery and metabolism. Similarly, CTC-derived models provide a sensitive platform for drug testing in advanced-stage patients, while also facilitating the development of drugs aimed at suppressing tumor metastasis. Meanwhile, the demand for large-scale testing has promoted the development of tumor organoids-on-chips, which serves as an optimal tool for high-throughput drug screening. This review summarizes the establishment and development of PDX, CTC-derived models, and tumor organoids-on-chips and addresses their distinctive advantages in drug discovery, sensitive testing, and screening, which demonstrate the potential to aid in the selection of suitable models for fundamental cancer research and clinical trials, and further developing the personalized drug therapy.
Keywords: Circulating tumor cell-derived model; Patient-derived xenograft model; Personalized drug therapy; Tumor organoids-on-chips.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Comparative analysis of response to treatments and molecular features of tumor-derived organoids versus cell lines and PDX derived from the same ovarian clear cell carcinoma.J Exp Clin Cancer Res. 2023 Oct 7;42(1):260. doi: 10.1186/s13046-023-02809-8. J Exp Clin Cancer Res. 2023. PMID: 37803448 Free PMC article.
-
Characterization of drug responses of mini patient-derived xenografts in mice for predicting cancer patient clinical therapeutic response.Cancer Commun (Lond). 2018 Sep 26;38(1):60. doi: 10.1186/s40880-018-0329-5. Cancer Commun (Lond). 2018. PMID: 30257718 Free PMC article.
-
Translational and Clinical Relevance of PDX-Derived Organoid Models in Oncology Drug Discovery and Development.Curr Protoc. 2022 Jul;2(7):e431. doi: 10.1002/cpz1.431. Curr Protoc. 2022. PMID: 35789132
-
Circulating tumor cell-derived organoids: Current challenges and promises in medical research and precision medicine.Biochim Biophys Acta Rev Cancer. 2018 Apr;1869(2):117-127. doi: 10.1016/j.bbcan.2017.12.005. Epub 2018 Jan 31. Biochim Biophys Acta Rev Cancer. 2018. PMID: 29360544 Free PMC article. Review.
-
Applications of patient-derived tumor xenograft models and tumor organoids.J Hematol Oncol. 2020 Jan 7;13(1):4. doi: 10.1186/s13045-019-0829-z. J Hematol Oncol. 2020. PMID: 31910904 Free PMC article. Review.
Cited by
-
Precision Targeting in Metastatic Prostate Cancer: Molecular Insights to Therapeutic Frontiers.Biomolecules. 2025 Apr 27;15(5):625. doi: 10.3390/biom15050625. Biomolecules. 2025. PMID: 40427518 Free PMC article. Review.
-
Establishment of Two Novel Ovarian Tumor Cell Lines with Characteristics of Mucinous Borderline Tumors or Dedifferentiated Carcinoma-Implications for Tumor Heterogeneity and the Complex Carcinogenesis of Mucinous Tumors.Cancers (Basel). 2025 May 20;17(10):1716. doi: 10.3390/cancers17101716. Cancers (Basel). 2025. PMID: 40427213 Free PMC article.
-
Mutational Patterns in Colorectal Cancer: Do PDX Models Retain the Heterogeneity of the Original Tumor?Int J Mol Sci. 2025 May 26;26(11):5111. doi: 10.3390/ijms26115111. Int J Mol Sci. 2025. PMID: 40507920 Free PMC article.
References
-
- Harrison R.K. Phase II and phase III failures: 2013–2015. Nat. Rev. Drug Discov. 2016;15(12):817–818. - PubMed
-
- De Martini D. Empowering phase II clinical trials to reduce phase III failures. Pharmaceut. Stat. 2020;19(3):178–186. - PubMed
-
- Podaza E., et al. Next generation patient derived tumor organoids. Transl. Res. 2022;250:84–97. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

