Mechanobiology in cellular, molecular, and tissue adaptation
- PMID: 40395638
- PMCID: PMC12082144
- DOI: 10.1016/j.mbm.2023.100022
Mechanobiology in cellular, molecular, and tissue adaptation
Abstract
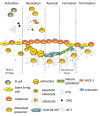
The use of mechanical biology and biomechanical signal transduction is a novel approach to attenuate biological tissue degeneration, whereas the understanding of specific cellular responses is critical to delineate the underlying mechanism. Dynamic mechanical signals with optimized loading signals, i.e., intensity and frequency, have been shown to have the potential to regulate adaptation and regeneration. Mechanotransduction pathways are of great interest in elucidating how mechanical signals produce such observed effects, including reduced tissue mass loss, increased healing and formation, and cell differentiation. While mechanobiology in the adaptation of cells and tissues is observed and recorded in the literature, its application in disease mechanism and treatment is under development. We would congratulate the opening of the Mechanobiology in Medicine journal, which provides an effective platform for advanced research in basic mechanotransduction and its translation in disease diagnosis, therapeutics, and beyond. This review aims to develop a cellular and molecular understanding of the mechanotransduction processes in tissue regeneration, which may provide new insights into disease mechanisms and the promotion of healing. Particular attention is allotted to the responses of mechanical loading, including potential cellular and molecular pathways, such as mechanotransduction associated with mechanotransduction pathways (e.g., Piezo ion channels and Wnt signaling), immune-response, neuron development, tissue adaptation and repair, and stem cell differentiation. Altogether, these discussed data highlight the complex yet highly coordinated mechanotransduction process in tissue regeneration.
Keywords: Biomechanics & mechanobiology; Bone adaptation; Cardiovascular adaptation; Cartilage regeneration; Loading frequency; Muscle atrophy; Osteopenia; Piezo 1; Ultrasound treatment.
© 2023 Published by Elsevier B.V. on behalf of Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

