Acute ischaemia and gap junction modulation modify propagation patterns across Purkinje-myocardial junctions
- PMID: 40395645
- PMCID: PMC12089142
- DOI: 10.3389/fphys.2025.1540400
Acute ischaemia and gap junction modulation modify propagation patterns across Purkinje-myocardial junctions
Abstract
Background: The Purkinje network is essential for normal electrical impulse propagation in the heart but has also been implicated in ventricular arrhythmias. Previous experimental work has suggested that not all Purkinje-myocardial junctions (PMJs) are active at rest due to source-sink mismatch at the PMJs.
Objective: We hypothesized that pathological conditions that cause gap junction uncoupling (e.g., acute ischaemia), would increase the number of active PMJs, leading to more complex activation patterns.
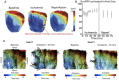
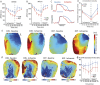
Methods: We investigated this using a whole-heart intact Purkinje system preparation that allowed direct high-resolution endocardial mapping to interrogate PMJ function. Twelve (7 control, five rotigaptide) Langendorff-perfused hearts from New Zealand white rabbits were subjected to an ischaemia-reperfusion protocol and optically mapped. Computational modelling was performed to determine the effects of gap junction coupling on PMJ function, and on the complexity of endocardial activation.
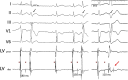
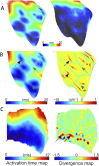
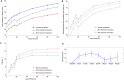
Results: During ischaemia, the percentage of right ventricle area activated within the first 5 ms decreased from baseline 62% ± 7% to 52% ± 8% during early ischaemia (p = 0.04), consistent with slowing of conduction. This was followed by a paradoxical increase in late-ischaemia (60% ± 8%) due to extra regions of early activation. Gap junction enhancement with rotigaptide during ischaemia abolished the aforementioned pattern. Parallel computational experiments replicated experimental findings only when the number of functional PMJs was increased during ischaemia. With more active PMJs, there were more breakthrough sites with increased complexity of activation, as also measured in biological preparations.
Conclusion: Normally-quiescent PMJs can become active in the context of gap junction uncoupling during acute ischaemia. Pharmacological gap junction modulation may alter propagation patterns across PMJs and may be used as a therapeutic strategy for Purkinje system associated arrhythmias.
Keywords: Purkinje-myocardial junction; cardiac electrophysiology; computer modelling; ischaemia; purkinje system.
Copyright © 2025 Jabbour, Behradfar, Debney, Nygren, Hartley, Efimov, Hocini, Peters, Ng and Vigmond.
Conflict of interest statement
Author EV was employed by IMB, UMR5251. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
The role of Purkinje-myocardial coupling during ventricular arrhythmia: a modeling study.PLoS One. 2014 Feb 7;9(2):e88000. doi: 10.1371/journal.pone.0088000. eCollection 2014. PLoS One. 2014. PMID: 24516576 Free PMC article.
-
A quantitative structural and morphometric analysis of the Purkinje network and the Purkinje-myocardial junctions in pig hearts.J Anat. 2017 May;230(5):664-678. doi: 10.1111/joa.12594. Epub 2017 Mar 3. J Anat. 2017. PMID: 28256093 Free PMC article.
-
Influence of the Purkinje-muscle junction on transmural repolarization heterogeneity.Cardiovasc Res. 2014 Sep 1;103(4):629-40. doi: 10.1093/cvr/cvu165. Epub 2014 Jul 3. Cardiovasc Res. 2014. PMID: 24997066 Free PMC article.
-
Pharmacological modulation of gap junction function with the novel compound rotigaptide: a promising new principle for prevention of arrhythmias.Basic Clin Pharmacol Toxicol. 2007 Oct;101(4):215-30. doi: 10.1111/j.1742-7843.2007.00123.x. Basic Clin Pharmacol Toxicol. 2007. PMID: 17845503 Review.
-
Ischaemia-induced cellular electrical uncoupling and ventricular fibrillation.Neth Heart J. 2002 Sep;10(9):360-365. Neth Heart J. 2002. PMID: 25696129 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

