Ultrasound Molecular Imaging of Blood Vessel Walls and Vulnerable Plaques via CXCR4-Targeted Nanoscale GVs
- PMID: 40395655
- PMCID: PMC12091238
- DOI: 10.2147/IJN.S504265
Ultrasound Molecular Imaging of Blood Vessel Walls and Vulnerable Plaques via CXCR4-Targeted Nanoscale GVs
Abstract
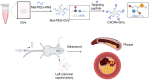
Purpose: C-X-C chemokine receptor 4 (CXCR4) mediates the inflammatory response of atherosclerotic vulnerable plaques (ASVP) and is a potential biomarker of atherosclerotic vulnerable plaques. The purpose of this study was to use the imaging ability of a new type of ultrasound contrast agent, nanoscale biosynthetic gas vesicles (GVs), on the vascular wall and to combine the specific ligand of CXCR4 to construct a targeted molecular probe to achieve early identification of atherosclerotic vulnerable plaques and guide clinical treatment decisions.
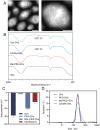
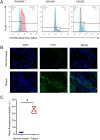
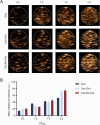
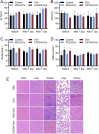
Materials and methods: Compared three contrast agents: GVs, the micro-contrast agent SonoVue, and polyethylene glycol (PEG)-modified GVs in the carotid artery. The expression of CXCR4 in atherosclerotic plaques was demonstrated using flow cytometry and immunofluorescence experiments. Cell adhesion and in vivo ultrasound imaging experiments demonstrated their ability to target the nanoscale biosynthetic gas vesicles. The safety of GVs, PEG-GVs, and CXCR4-GVs was tested the CCk8 test, H&E staining, and serum detection.
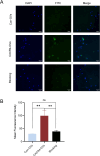
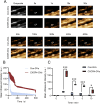
Results: Strong CXCR4 expression was observed in plaques, whereas little expression was observed in normal vessels. GVs can produce stable contrast signals on the carotid artery walls of rats, whereas PEG-GVs can produce more lasting contrast signals on the carotid artery wall of rats. CXCR4-GVs exhibited excellent binding capability to ox-LDL-induced RAW264.7 cells. Animal experiments showed that compared with Con-GVs, CXCR4-GVs injected plaque imaging signal was stronger and more durable. In vitro scanning of vulnerable plaques in rats injected with fluorescent vesicles demonstrated that CXCR4-GVs oozed through the neovasculars within vulnerable plaques and aggregated in vulnerable plaques. Through the CCK8 test, H&E staining, and serum detection, the safety of CXCR4-GVs was confirmed.
Conclusion: CXCR4-GVs were constructed as targeted molecular probes, which can be proven to have good targeting properties to vulnerable atherosclerotic plaques.
Keywords: CXCR4; nanoscale biosynthetic gas vesicles; ultrasound molecular imaging; vulnerable plaque.
© 2025 Lin et al.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this study.
Figures


Similar articles
-
Molecular imaging diagnosis of atherosclerotic vulnerable plaque in rabbit carotid artery using a self-assembled nanoscale ultrasound microbubble contrast agent.Rev Cardiovasc Med. 2021 Dec 22;22(4):1657-1666. doi: 10.31083/j.rcm2204173. Rev Cardiovasc Med. 2021. PMID: 34957808
-
Targeted Molecular Iron Oxide Contrast Agents for Imaging Atherosclerotic Plaque.Nanotheranostics. 2020 May 30;4(4):184-194. doi: 10.7150/ntno.44712. eCollection 2020. Nanotheranostics. 2020. PMID: 32637296 Free PMC article.
-
Ultrasound Molecular Imaging of Bladder Cancer via Extradomain B Fibronectin-Targeted Biosynthetic GVs.Int J Nanomedicine. 2023 Aug 29;18:4871-4884. doi: 10.2147/IJN.S412422. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37662687 Free PMC article.
-
Molecular imaging of carotid plaque vulnerability.Cerebrovasc Dis. 2015;39(1):5-12. doi: 10.1159/000369123. Epub 2014 Dec 24. Cerebrovasc Dis. 2015. PMID: 25547782 Review.
-
Molecular magnetic resonance imaging of atherosclerotic vessel wall disease.Eur Radiol. 2016 Mar;26(3):910-20. doi: 10.1007/s00330-015-3881-2. Epub 2015 Jul 3. Eur Radiol. 2016. PMID: 26139316 Review.
References
-
- Piepoli MF, Hoes AW, Hoes AW; Authors/Task Force Members, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis. 2016;252:207–274. doi:10.1016/j.atherosclerosis.2016.05.037 - DOI - PubMed
-
- Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5(1):56. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

