Efficacy of mavacamten in patients with hypertrophic cardiomyopathy and mid-ventricular obstruction: case series
- PMID: 40395679
- PMCID: PMC12090046
- DOI: 10.1093/ehjcr/ytaf229
Efficacy of mavacamten in patients with hypertrophic cardiomyopathy and mid-ventricular obstruction: case series
Abstract
Background: Mavacamten is a cardiac-specific myosin inhibitor approved for treatment of adults with hypertrophic cardiomyopathy (HCM) symptomatic for left ventricular outflow tract (LVOT) obstruction. Since obstruction is favoured by a hyper-contractile state, it would be logical to suppose that mavacamten may also be effective in patients with mid-ventricular obstruction (MVO). We present our experience with two HCM patients having MVO effectively treated with mavacamten.
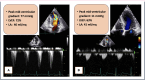
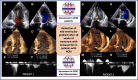
Case summary: The first case is a 55-year-old woman presenting with dyspnoea and exertional fatigue, with obstructive HCM (HOCM) and mid-ventricular peak gradient of 77 mmHg associated with LVOT obstruction. The treatment with mavacamten 5 mg daily determined relief of symptoms. At 16-week follow-up, there was a significant reduction of peak gradient (11 mmHg in mid-ventricular tract) and a significant decrease in NT-proBNP levels from 1287 to 178 ng/L. The second case is a 55-year-old woman with predominant mid-ventricular HOCM (peak gradient 52 mmHg) and past history of septal myectomy, with a residual significant gradient measured at LVOT level. The patient was started on mavacamten 5 mg daily, subsequently up-titrated to 10 mg. At 16-week follow-up, there was a significant reduction of peak gradient to 10 mmHg and a significant decrease in NT-proBNP levels from 3910 to 718 ng/L.
Discussion: These two cases highlight the efficacy of mavacamten in the reduction of MVO, suggesting that it may be a valid therapeutic option also in patients with isolated MVO, frequently more difficult to be adequately treated.
Keywords: Case report; Hypertrophic cardiomyopathy; Mavacamten; Mid-ventricular obstruction.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest. L.C. was part of the advisory board of BMS for mavacamten.
Figures

References
-
- Olivotto I, Oreziak A, Barriales-Villa R, Abraham TP, Masri A, Garcia-Pavia P, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020;396:759–769. - PubMed
-
- Desai MY, Owens A, Geske JB, Wolski K, Naidu SS, Smedira NG, et al. Myosin inhibition in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy (VALOR HCM). J Am Coll Cardiol 2022;80:95–108. - PubMed
-
- Hegde SM, Lester SJ, Solomon SD, Michels M, Elliott PM, Nagueh SF, et al. Effect of mavacamten on echocardiographic features in symptomatic patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2021;78:2518–2532. - PubMed
-
- Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur Heart J 2023;44:3503–3626. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
