Amaranthus spinosus Linn. Extract as an Innovative Strategy to Regulate Biomarkers for Ovarian Hyperthecosis via Circular RNA (hsa-circ-0001577): Evidence From Biochemical, Metabolomics, Histological, and Phytochemical Profiling
- PMID: 40395717
- PMCID: PMC12091212
- DOI: 10.1002/fsn3.70314
Amaranthus spinosus Linn. Extract as an Innovative Strategy to Regulate Biomarkers for Ovarian Hyperthecosis via Circular RNA (hsa-circ-0001577): Evidence From Biochemical, Metabolomics, Histological, and Phytochemical Profiling
Abstract
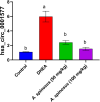
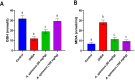
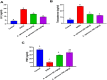
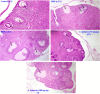
Amaranthus species, including A. spinosus Linn, are well-known vegetables whose leaves, shoots, fragile stems, and grains are commonly utilized as herbs in soups or sauces, aside from traditional uses to treat a wide range of illnesses. Ovarian hyperthecosis is a common syndrome associated with metabolomics and endocrinology that lowers female fertility. The investigation of novel biomarkers and targeted therapies for the detection and treatment of ovarian hyperthecosis is of interest. Types of noncoding RNAs known as circular RNAs (circRNAs) have covalently closed cyclic structures, are widely distributed, and exhibit expression patterns that are particular to different stages of development. Ovarian hyperthecosis was induced in rats via dehydroepiandrosterone (DHEA) followed by 1 month of treatment with 50 and 100 mg/kg of the A. spinosus EtOH extract. Further, oxidative stress biomarkers including GSH and MDA were investigated in addition to hormonal biomarkers, such as Luteinizing hormone and testosterone hormone, a metabolomics approach modeled using orthogonal partial least squares discriminant analysis (OPLS-DA), and circRNA (hsa-circ-0001577). Furthermore, UHPLC-ESI-Orbitrap-MS analysis was used for metabolites profiling to identify active agents in the plant extract. Results revealed a significant improvement in these biomarkers in the DHEA group treated with A. spinosus, especially at high doses, and further confirmed via histopathological assays. Multivariate data analyses of serum metabolome indicated significant variations in serum profiles among normal, disease, and treated groups. Variable importance in the projection (VIP) values guided the selection of differentiated metabolites, revealing significant changes in metabolite concentrations. UHPLC-ESI-Orbitrap-MS analysis identified 72 bioactive metabolites belonging to phenolics, triterpenoidal saponins, and pyridines In conclusion, A. spinosus could be a management approach for ovarian hyperthecosis therapy via regulating circRNA (hsa-circ-0001577), disturbed hormonal balance, and metabolomics biomarkers based assays.
Keywords: Amaranthus spinosus; circular RNAs; metabolomics; ovarian hyperthecosis; phytochemical profile.
© 2025 The Author(s). Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
[Pseudotargeted metabolomics analysis of pine pollen intervention in the liver of premature ovarian failure rats].Se Pu. 2023 Jan;41(1):47-57. doi: 10.3724/SP.J.1123.2022.04017. Se Pu. 2023. PMID: 36633076 Free PMC article. Chinese.
-
Plasma circRNA microarray profiling identifies novel circRNA biomarkers for the diagnosis of ovarian cancer.J Ovarian Res. 2022 May 12;15(1):58. doi: 10.1186/s13048-022-00988-0. J Ovarian Res. 2022. PMID: 35550610 Free PMC article.
-
[Plasma metabolomics in a deep vein thrombosis rat model based on ultra-high performance liquid chromatography-electrostatic field orbitrap high resolution mass spectrometry].Se Pu. 2022 Aug;40(8):736-745. doi: 10.3724/SP.J.1123.2021.12024. Se Pu. 2022. PMID: 35903841 Free PMC article. Chinese.
-
Translational Metabolomics of Head Injury: Exploring Dysfunctional Cerebral Metabolism with Ex Vivo NMR Spectroscopy-Based Metabolite Quantification.In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 25. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 25. PMID: 26269925 Free Books & Documents. Review.
-
Novel potential tumor biomarkers: Circular RNAs and exosomal circular RNAs in gastrointestinal malignancies.J Clin Lab Anal. 2020 Jul;34(7):e23359. doi: 10.1002/jcla.23359. Epub 2020 May 17. J Clin Lab Anal. 2020. PMID: 32419229 Free PMC article. Review.
References
-
- Abbott, D. H. , Greinwald E. P., and Levine J. E.. 2022. “Chapter 3‐De velopmental Origins of Polycystic Ovary Syndrome: Everything Starts In Utero.” Polycystic Ovary Syndrome: 23–38. 10.1016/B978-0-12-823045-9.00009-2. - DOI
-
- Abruzzese, G. A. , Silva A. F., Velazquez M. E., Ferrer M. J., and Motta A. B.. 2022. “Hyperandrogenism and Polycystic Ovary Syndrome: Effects in Pregnancy and Offspring Development.” WIREs Mechanisms of Disease 14, no. 5: e1558. - PubMed
-
- Akinloye, O. , Sulaimon L., Ogunbiyi O., et al. 2023. “ Amaranthus spinosus (Spiny Pigweed) Methanol Leaf Extract Alleviates Oxidative and Inflammation Induced by Doxorubicin in Male Sprague Dawley Rats.” Advances in Traditional Medicine 23, no. 4: 1231–1248.
LinkOut - more resources
Full Text Sources

