Clinical insights into IL-23 inhibition: risankizumab for Crohn's disease through a systematic review and meta-analysis of randomized controlled trials
- PMID: 40395763
- PMCID: PMC12089713
- DOI: 10.1177/17562848251338743
Clinical insights into IL-23 inhibition: risankizumab for Crohn's disease through a systematic review and meta-analysis of randomized controlled trials
Abstract
Background and aims: Crohn's disease is a chronic inflammatory disorder with rising global prevalence, marked by abdominal pain, diarrhea, and fatigue. Interleukin (IL)-23 plays a pivotal role in Crohn's disease pathogenesis, making it a therapeutic target. Risankizumab, a monoclonal antibody targeting the IL-23 p19 subunit, has shown potential in clinical trials.
Objectives: This meta-analysis evaluates the efficacy and safety of Risankizumab in achieving clinical remission, clinical response, and endoscopic remission in patients with moderate-to-severe Crohn's disease.
Design: A systematic review and meta-analysis were conducted following PRISMA 2020 guidelines.
Data sources and methods: A comprehensive search of PubMed, Embase, Cochrane CENTRAL, Web of Science, and ClinicalTrials.gov was performed to identify randomized controlled trials (RCTs) assessing Risankizumab in Crohn's disease. Primary outcomes were clinical remission, clinical response, and endoscopic remission, with secondary outcomes focusing on treatment-related adverse events. A random-effects model estimated odds ratios (ORs) with 95% confidence intervals. Meta-regression analyzed dose- and duration-dependent effects.
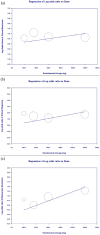
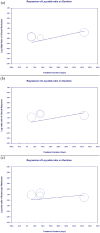
Results: Four RCTs involving 1774 participants showed that Risankizumab significantly improved clinical remission (OR = 2.223), clinical response (OR = 2.483), and endoscopic remission (OR = 4.112). Dose-dependent improvements were observed, with treatment duration affecting clinical remission (p = 0.0158) but not clinical response or endoscopic remission. Adverse event rates were comparable between Risankizumab and placebo groups (OR = 0.872, p = 0.592).
Conclusion: Risankizumab is effective in achieving clinical and endoscopic outcomes in moderate-to-severe Crohn's disease, demonstrating dose-dependent benefits and a favorable safety profile, supporting its use as a therapeutic option. However, the limited number of studies may affect the robustness of these findings. Further large-scale RCTs are needed to validate its long-term efficacy, safety in elderly populations, and effectiveness in biologic-naïve patients.
Trial registration: This systematic review and meta-analysis were registered with the INPLASY database under registration number INPLASY202530014. The full protocol is accessible at DOI: 10.37766/inplasy2025.3.0014.
Keywords: Crohn’s disease; IL-23 inhibitors; Risankizumab; clinical remission; endoscopic remission; meta-analysis.
Plain language summary
How Risankizumab helps treat Crohn’s disease: a review of clinical trial results Crohn’s disease is a long-term condition that causes inflammation in the digestive system. People with Crohn’s disease often experience symptoms like stomach pain, diarrhea, and tiredness, which can significantly affect their daily lives. Researchers have found that a protein called interleukin-23 (IL-23) plays a key role in this disease. Targeting IL-23 may help control the inflammation. Risankizumab is a medicine designed to block a part of IL-23, and it has been tested in clinical trials to see if it can help people with moderate-to-severe Crohn’s disease. To understand how well it works and whether it is safe, we combined the results of multiple high-quality studies in a process called a metaanalysis. Our analysis included four studies with a total of 1,774 patients. We found that Risankizumab significantly improved three key outcomes: Clinical remission: Fewer or no symptoms of Crohn’s disease. Clinical response: Noticeable improvement in symptoms. Endoscopic remission: Healing of the digestive tract, confirmed by a camera test. Patients who received Risankizumab were more likely to achieve these outcomes compared to those who received a placebo. The benefits were stronger with higher doses of the medication and with longer treatment durations for clinical remission. Importantly, the medicine was found to be safe. The rates of side effects were similar between patients taking Risankizumab and those taking a placebo. In conclusion, Risankizumab is an effective and safe treatment for people with moderate-to-severe Crohn’s disease. It helps reduce symptoms, promotes healing in the digestive tract, and works better at higher doses. This makes it a promising option for managing this challenging condition.
© The Author(s), 2025.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures



Similar articles
-
Risankizumab as maintenance therapy for moderately to severely active Crohn's disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial.Lancet. 2022 May 28;399(10340):2031-2046. doi: 10.1016/S0140-6736(22)00466-4. Lancet. 2022. PMID: 35644155 Clinical Trial.
-
Risankizumab in patients with moderate to severe Crohn's disease: an open-label extension study.Lancet Gastroenterol Hepatol. 2018 Oct;3(10):671-680. doi: 10.1016/S2468-1253(18)30233-4. Epub 2018 Jul 25. Lancet Gastroenterol Hepatol. 2018. PMID: 30056030 Clinical Trial.
-
Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn's disease: a randomised, double-blind, placebo-controlled phase 2 study.Lancet. 2017 Apr 29;389(10080):1699-1709. doi: 10.1016/S0140-6736(17)30570-6. Epub 2017 Apr 12. Lancet. 2017. PMID: 28411872 Clinical Trial.
-
One-Year Efficacy of Guselkumab Versus Advanced Therapies for the Treatment of Moderately to Severely Active Crohn's Disease: A Network Meta-Analysis.Adv Ther. 2025 Jun;42(6):2708-2727. doi: 10.1007/s12325-025-03183-x. Epub 2025 May 6. Adv Ther. 2025. PMID: 40327280 Free PMC article. Review.
-
Anti-IL-12/23p40 antibodies for maintenance of remission in Crohn's disease.Cochrane Database Syst Rev. 2019 Dec 12;12(12):CD012804. doi: 10.1002/14651858.CD012804.pub2. Cochrane Database Syst Rev. 2019. PMID: 31828765 Free PMC article.
References
-
- Torres J, Mehandru S, Colombel J-F, et al.. Crohn’s disease. Lancet 2017; 389(10080): 1741–1755. - PubMed
-
- van der Have M, van der Aalst KS, Kaptein AA, et al.. Determinants of health-related quality of life in Crohn’s disease: a systematic review and meta-analysis. J Crohns Colitis 2014; 8(2): 93–106. - PubMed
-
- Sarra M, Pallone F, Macdonald TT, et al.. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis 2010; 16(10): 1808–1813. - PubMed
LinkOut - more resources
Full Text Sources

