Flavonoid carbamate hybrids: design, synthesis, and evaluation as multi-target enzyme inhibitors for Alzheimer's disease
- PMID: 40395797
- PMCID: PMC12090193
- DOI: 10.1039/d5ra02267c
Flavonoid carbamate hybrids: design, synthesis, and evaluation as multi-target enzyme inhibitors for Alzheimer's disease
Abstract
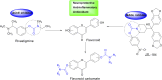
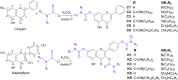
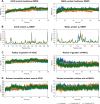
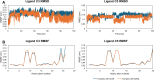
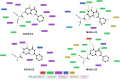
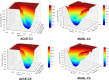
Alzheimer's disease is characterized by cholinergic dysfunction and neuroinflammation, with acetylcholinesterase and monoacylglycerol lipase emerging as important therapeutic targets. In this study, a series of novel flavonoid carbamate derivatives were synthesized from chrysin and kaempferol, and their structures were confirmed via NMR and HRMS spectroscopy. The inhibitory activities of these compounds were evaluated against acetylcholinesterase and monoacylglycerol lipase using in vitro enzymatic assays. Among them, C3 and C5 exhibited significant dual inhibition, with IC50 values of 22.86 μM and 46.65 μM for monoacylglycerol lipase, and 61.78 μM and 89.40 μM for acetylcholinesterase, respectively. Molecular docking studies revealed key binding interactions, while molecular dynamics simulations demonstrated their stability within the active sites of target enzymes. These findings highlight C3 and C5 as promising candidates for further investigation in the development of dual acetylcholinesterase/monoacylglycerol lipase inhibitors for Alzheimer's disease treatment.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
"Triazole-linked thiazolidinedione-Benzothiazole hybrids: Design and biological evaluation as AChE inhibitors".Bioorg Chem. 2025 Apr;157:108295. doi: 10.1016/j.bioorg.2025.108295. Epub 2025 Feb 20. Bioorg Chem. 2025. PMID: 40010133
-
Synthesis, design, and cholinesterase inhibitory activity of novel 1,2,4-triazole Schiff bases: A combined experimental and computational approach.Int J Biol Macromol. 2025 May;306(Pt 1):141350. doi: 10.1016/j.ijbiomac.2025.141350. Epub 2025 Feb 20. Int J Biol Macromol. 2025. PMID: 39986523
-
Discovery of Novel Coumarin-Schiff Base Hybrids as Potential Acetylcholinesterase Inhibitors: Design, Synthesis, Enzyme Inhibition, and Computational Studies.Pharmaceuticals (Basel). 2023 Jul 6;16(7):971. doi: 10.3390/ph16070971. Pharmaceuticals (Basel). 2023. PMID: 37513883 Free PMC article.
-
Promising thiazolidinedione-thiazole based multi-target and neuroprotective hybrids for Alzheimer's disease: Design, synthesis, in-vitro, in-vivo and in-silico studies.Eur J Med Chem. 2025 Apr 5;287:117327. doi: 10.1016/j.ejmech.2025.117327. Epub 2025 Feb 3. Eur J Med Chem. 2025. PMID: 39914143
-
Multi-targeted benzylpiperidine-isatin hybrids: Design, synthesis, biological and in silico evaluation as monoamine oxidases and acetylcholinesterase inhibitors for neurodegenerative disease therapies.J Comput Aided Mol Des. 2025 Feb 28;39(1):10. doi: 10.1007/s10822-025-00588-2. J Comput Aided Mol Des. 2025. PMID: 40021503
References
-
- Singh S. Mahajan M. Kumar D. Singh K. Chowdhary M. Amit Neuropeptides. 2023;102:102369. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

