HS-GC-MS Method for the Diagnosis of IBD Dynamics in a Model of DSS-Induced Colitis
- PMID: 40395844
- PMCID: PMC12086341
- DOI: 10.21769/BioProtoc.5246
HS-GC-MS Method for the Diagnosis of IBD Dynamics in a Model of DSS-Induced Colitis
Abstract
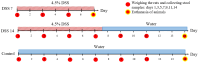
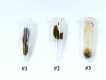
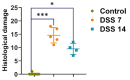
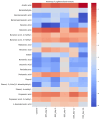
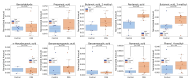
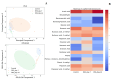
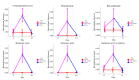
Inflammatory bowel disease (IBD) is highly prevalent globally and, in the majority of cases, remains asymptomatic during its initial stages. The gastrointestinal microbiota secretes volatile organic compounds (VOCs), and their composition alters in IBD. The examination of VOCs could prove beneficial in complementing diagnostic techniques to facilitate the early identification of IBD risk. In this protocol, a model of sodium dextran sulfate (DSS)-induced colitis in rats was successfully implemented for the non-invasive metabolomic assessment of different stages of inflammation. Headspace-gas chromatography-mass spectrometry (HS-GC-MS) was used as a non-invasive method for inflammation assessment at early and remission stages. The disease activity index (DAI) and histological method were employed to assess intestinal inflammation. The HS-GC-MS method demonstrated high sensitivity to intestine inflammation, confirmed by DAI and histology assay, in the acute and remission stages, identifying changes in the relative content of VOCs in stools. HS-GC-MS may be a useful and non-invasive method for IBD diagnostics and therapy effectiveness control. Key features • Experiments performed in vivo to better control DSS-induced colon damage and to aid the study of IBD development in humans. • Optimized for the following organisms: Wistar rats and C57BL/6 mice. • An easily assessed disease activity index (DAI) (weight loss, stool consistency, degree of fecal occult blood) and histopathological examination are suggested for additional IBD confirmation. • Enables VOC testing with relatively small stool samples.
Keywords: DSS-induced colitis; Headspace–gas chromatography–mass spectrometry (HS–GC–MS); IBD; Metabolomic profile; Volatile organic compounds (VOCs).
©Copyright : © 2025 The Authors; This is an open access article under the CC BY-NC license.
Conflict of interest statement
Competing interestsThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









Similar articles
-
GC-MS with Headspace Extraction for Non-Invasive Diagnostics of IBD Dynamics in a Model of DSS-Induced Colitis in Rats.Int J Mol Sci. 2024 Mar 14;25(6):3295. doi: 10.3390/ijms25063295. Int J Mol Sci. 2024. PMID: 38542269 Free PMC article.
-
Bacteroides vesicles promote functional alterations in the gut microbiota composition.Microbiol Spectr. 2024 Nov 5;12(11):e0063624. doi: 10.1128/spectrum.00636-24. Epub 2024 Sep 30. Microbiol Spectr. 2024. PMID: 39345205 Free PMC article.
-
Gut bacteria Prevotellaceae related lithocholic acid metabolism promotes colonic inflammation.J Transl Med. 2025 Jan 13;23(1):55. doi: 10.1186/s12967-024-05873-6. J Transl Med. 2025. PMID: 39806416 Free PMC article.
-
Investigating intestinal inflammation in DSS-induced model of IBD.J Vis Exp. 2012 Feb 1;(60):3678. doi: 10.3791/3678. J Vis Exp. 2012. PMID: 22331082 Free PMC article.
-
[Recent advances in the application of headspace gas chromatography-mass spectrometry].Se Pu. 2018 Oct 8;36(10):962-971. doi: 10.3724/SP.J.1123.2018.05013. Se Pu. 2018. PMID: 30378354 Review. Chinese.
References
-
- Adamkova P., Hradicka P., Kupcova Skalnikova H., Cizkova V., Vodicka P., Farkasova Iannaccone S., Kassayova M., Gancarcikova S. and Demeckova V.(2022). Dextran Sulphate Sodium Acute Colitis Rat Model: A Suitable Tool for Advancing Our Understanding of Immune and Microbial Mechanisms in the Pathogenesis of Inflammatory Bowel Disease. Vet Sci. 9(5): 238 10.3390/vetsci9050238 - DOI - PMC - PubMed
-
- Wang B., Yao M., Lv L., Ling Z. and Li L.(2017). The Human Microbiota in Health and Disease. Engineering. 3(1): 71 82 82. 10.1016/J.ENG .2017.01.008 - DOI
-
- Niccolai E., Baldi S., Ricci F., Russo E., Nannini G., Menicatti M., Poli G., Taddei A., Bartolucci G., Calabrò A. S., et al. .(2019). Evaluation and comparison of short chain fatty acids composition in gut diseases. World J Gastroenterol. 25(36): 5543 5558 5558. 10.3748/wjg.v25 .i36.5543 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

