Generation of FECD Phenotypes in the Mouse Cornea by UVA Exposure and Surgical Removal of its Corneal Endothelial Layer
- PMID: 40395849
- PMCID: PMC12086347
- DOI: 10.21769/BioProtoc.5249
Generation of FECD Phenotypes in the Mouse Cornea by UVA Exposure and Surgical Removal of its Corneal Endothelial Layer
Abstract
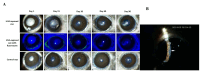
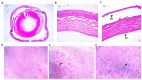
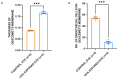
Fuchs endothelial corneal dystrophy (FECD) is a rare and multifactorial disorder leading to cell death in the innermost layer of the cornea, i.e., the endothelium; UV radiation is reported as the major environmental risk for the disease. Establishing an animal model for this disease has remained challenging in FECD research. We have developed a detailed protocol for the establishment of a UVA-induced FECD mouse model and removal of corneal endothelium from the eye for further molecular and histological studies by taking references from previous studies. UVA light of 500 J/cm2 was focused on the C57BL/6J female mouse cornea and kept for an observation period of 90 days. The animal developed corneal scarring by the end of three months. Slit-lamp microscopy and alizarin red-trypan blue staining confirmed endothelial cell death and formation of corneal guttae in the endothelium. Surgical removal of the endothelial layer was successfully done in the diseased mouse, and the result was confirmed by immunofluorescence. This study is relevant for in-depth research using a FECD mouse model, which will surpass the limitation of human tissue scarcity and can be used for in vivo drug targeting to develop therapeutics to cure FECD. Key features • UVA radiation induces FECD only in the exposed eye of female mice. • Females are more affected and develop the FECD phenotype. • This protocol will help dissect the endothelium layer with Descemet's membrane (DM) from the mouse cornea, which is equivalent to human surgical tissue.
Keywords: Cornea; Corneal endothelium; Dissection; Fuchs dystrophy; Mouse model; Slit-lamp microscopy; UVA irradiation.
©Copyright : © 2025 The Authors; This is an open access article under the CC BY-NC license.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures





Similar articles
-
Rapid detection of guttae area using aniline blue staining in Fuchs endothelial corneal dystrophy mouse model.Clin Exp Pharmacol Physiol. 2024 Oct;51(10):e13921. doi: 10.1111/1440-1681.13921. Clin Exp Pharmacol Physiol. 2024. PMID: 39223829
-
MitoQ relieves mitochondrial dysfunction in UVA and cigarette smoke-induced Fuchs endothelial corneal dystrophy.Exp Eye Res. 2024 Oct;247:110056. doi: 10.1016/j.exer.2024.110056. Epub 2024 Aug 22. Exp Eye Res. 2024. PMID: 39179169
-
TCF4 trinucleotide repeat expansions and UV irradiation increase susceptibility to ferroptosis in Fuchs endothelial corneal dystrophy.Redox Biol. 2024 Nov;77:103348. doi: 10.1016/j.redox.2024.103348. Epub 2024 Sep 10. Redox Biol. 2024. PMID: 39332053 Free PMC article.
-
The soil and the seed: The relationship between Descemet's membrane and the corneal endothelium.Exp Eye Res. 2023 Feb;227:109376. doi: 10.1016/j.exer.2022.109376. Epub 2022 Dec 30. Exp Eye Res. 2023. PMID: 36592681 Free PMC article. Review.
-
Fuchs endothelial corneal dystrophy: The vicious cycle of Fuchs pathogenesis.Prog Retin Eye Res. 2021 Jan;80:100863. doi: 10.1016/j.preteyeres.2020.100863. Epub 2020 May 8. Prog Retin Eye Res. 2021. PMID: 32438095 Free PMC article. Review.
References
-
- Elhalis H., Azizi B. and Jurkunas U. V.(2010). Fuchs endothelial corneal dystrophy. Ocul Surf. 8:173 84 84 . https://www.ncbi.nlm.nih.gov/books/NBK545248/ - PMC - PubMed
LinkOut - more resources
Full Text Sources

