Force-dependent rapid immunoassay of high specificity and sensitivity
- PMID: 40395855
- PMCID: PMC12082306
- DOI: 10.1016/j.mbm.2024.100061
Force-dependent rapid immunoassay of high specificity and sensitivity
Abstract
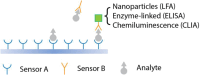
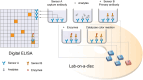
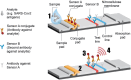
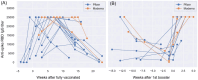
The significance of early detection and isolation of infected individuals, along with the quantitative assessment of antibodies against the virus, has gained widespread recognition during the ongoing covid-19 pandemic. This necessitates the development of cost-effective, user-friendly, decentralized testing methods characterized by both high sensitivity and specificity. In this article, we present a comprehensive review of an innovative, low-cost rapid decentralized immunoassay technology, applicable across various diagnostic and quantitative testing scenarios. Distinguishing itself from conventional immunoassay technologies, this method is featured with mechanically enhanced specificity without compromising sensitivity. We delve into the basic principle of the technology and a comparative analysis of this technology in relation to other immunodiagnostic methods, highlighting its potential applications in a wide spectrum of diagnostic tests.
Keywords: Antibody quantification; COVID-19; Finger-prick whole blood; Immunodiagnostics; Point-of-care (POC).
© 2024 Published by Elsevier B.V. on behalf of Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Rapid Quantitative Point-Of-Care Diagnostic Test for Post COVID-19 Vaccination Antibody Monitoring.Microbiol Spectr. 2022 Apr 27;10(2):e0039622. doi: 10.1128/spectrum.00396-22. Epub 2022 Mar 31. Microbiol Spectr. 2022. PMID: 35357223 Free PMC article.
-
Recent Advances in Quantum Dot-Based Lateral Flow Immunoassays for the Rapid, Point-of-Care Diagnosis of COVID-19.Biosensors (Basel). 2023 Aug 3;13(8):786. doi: 10.3390/bios13080786. Biosensors (Basel). 2023. PMID: 37622872 Free PMC article. Review.
-
SERS Based Lateral Flow Immunoassay for Point-of-Care Detection of SARS-CoV-2 in Clinical Samples.ACS Appl Bio Mater. 2021 Apr 19;4(4):2974-2995. doi: 10.1021/acsabm.1c00102. Epub 2021 Mar 17. ACS Appl Bio Mater. 2021. PMID: 35014387 Review.
-
Evaluation of performance of two SARS-CoV-2 Rapid IgM-IgG combined antibody tests on capillary whole blood samples from the fingertip.PLoS One. 2020 Sep 17;15(9):e0237694. doi: 10.1371/journal.pone.0237694. eCollection 2020. PLoS One. 2020. PMID: 32941461 Free PMC article.
-
Development of chemiluminescent lab-on-fiber immunosensors for rapid point-of-care testing of anti-SARS-CoV-2 antibodies and evaluation of longitudinal immune response kinetics following three-dose inactivation virus vaccination.J Med Virol. 2023 Jan;95(1):e28190. doi: 10.1002/jmv.28190. Epub 2022 Oct 10. J Med Virol. 2023. PMID: 36180404 Free PMC article.
References
-
- Sunkara V., Kumar S., Del Río J.S., Kim I., Cho Y.K. Lab-on-a-Disc for point-of-care infection diagnostics. Accounts Chem Res. 2021;54(19):3643–3655. - PubMed
-
- Syedmoradi L., Daneshpour M., Alvandipour M., Gomez F.A., Hajghassem H., Omidfar K. Point of care testing: the impact of nanotechnology. Biosens Bioelectron. 2017;87:373–387. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

