Generation and functional characterization of tuft cells in non-human primate pancreatic ducts through organoid culture systems
- PMID: 40395933
- PMCID: PMC12089129
- DOI: 10.3389/fcell.2025.1593226
Generation and functional characterization of tuft cells in non-human primate pancreatic ducts through organoid culture systems
Abstract
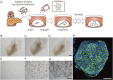
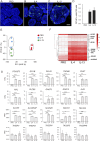
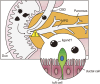
The pancreatic duct plays a key role in collecting pancreatic juice, which is rich in digestive enzymes. The fluid flows unidirectionally into the duodenum, where it mixes with partially digested food to further facilitate digestion. In this study, we report the generation of pancreatic ductal organoids from non-human primates for the first time, aimed at investigating the role of tuft cells that reside in the pancreatic duct since no studies have addressed the role of tuft cells in the pancreas. The organoids were maintained in a medium supplemented with Wnt3a, Noggin, R-spondin, and other factors that support pancreatic duct proliferation. These pancreatic organoids expressed the stem cell marker LGR5 mRNA and the ductal marker protein CK19, although tuft cell markers were not detectable at this stage. Upon stimulation with IL-4/13, tuft cell differentiation was confirmed by immunohistochemistry and transcriptomic analysis. We observed induction of DCLK1, as well as taste signaling molecules such as TRPM5 and PLCβ2, which are markers of type II taste cells. Additionally, upregulation of LYZ and DEFB1 mRNA indicated the expression of antimicrobial peptide markers, alongside molecules associated with inflammation. Furthermore, the differentiated organoids specifically responded to a bitter compound, suggesting that pancreatic tuft cells may play a role in detecting potentially harmful chemicals. Finally, immunohistochemical analysis identified tuft cells in the non-human primate pancreas, supporting their involvement in sensing harmful compounds and regulating protective responses within the pancreas.
Keywords: organoid; pancreas; primate; tuft cells; type 2 immunity.
Copyright © 2025 Sakaguchi, Kimura-Nakajima, Inaba, Hatano, Ogawa, Koshiishi, Tanaka, Kometani, Ohmoto, Sato, Imai and Iwatsuki.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

