Effects of Bariatric Surgery-Related Weight Loss on the Characteristics, Metabolism, and Immunomodulation of Adipose Stromal/Stem Cells in a Follow-Up Study
- PMID: 40395977
- PMCID: PMC12092157
- DOI: 10.1155/sci/1212255
Effects of Bariatric Surgery-Related Weight Loss on the Characteristics, Metabolism, and Immunomodulation of Adipose Stromal/Stem Cells in a Follow-Up Study
Abstract
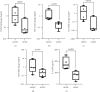
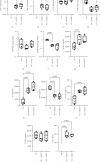
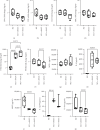
Background: The success of adipose stromal/stem cell (ASC)-based therapies may depend on donor characteristics such as body mass index (BMI). A high BMI may negatively impact the therapeutic potential of ASCs, but the effects of weight loss on ASC-mediated immunoregulation have not been extensively studied. Methods: ASCs were obtained from donors with obesity (obASCs) undergoing bariatric surgery and from the same donors after weight loss (wlASCs). Plasma samples, adipose tissue histology, and ASC characteristics, such as mitochondrial respiration and inflammatory factors, were studied before and after weight loss. The immunomodulatory capacity of ob/wlASCs was evaluated in cocultures with prepolarized and preactivated proinflammatory (M1) and anti-inflammatory (M2) macrophages by determining macrophage surface markers, gene expression, and cytokine secretion. Results: Weight loss significantly decreased plasma leptin levels and increased adiponectin levels. After weight loss, crown-like structures (CLSs) were undetectable, and the adipocyte size decreased. Weight loss significantly improved mitochondrial respiration in ASCs and resulted in a notable increase in their proliferative capacity. The proinflammatory marker genes tumor necrosis factor alpha (TNF-α), chemokine ligand 5 (CCL5), and cyclooxygenase-2 (COX2), as well as the proinflammatory cytokine interleukin 12p70 (IL-12p70), were significantly downregulated, while the anti-inflammatory gene tumor necrosis factor-inducible gene 6 (TSG6) was also significantly downregulated in ASC monocultures after weight loss. Following weight loss, ASCs exhibited increased proinflammatory properties when cocultured with macrophages, characterized by the downregulation of anti-inflammatory factors, along with the upregulation of several proinflammatory factors, compared with the effects of macrophage monocultures. Conversely, wlASCs demonstrated improved immunosuppressive functions in coculture with macrophages, as indicated by the upregulation of TSG6 gene expression and interleukin 4 (IL-4) secretion. Conclusions: Weight loss improved donors' metabolic health and partially recovered ASCs' anti-inflammatory gene expression and cytokine secretion profiles in monocultures. However, it was inadequate to fully restore the immunosuppressive functions of ASCs in cocultures with macrophages. Therefore, not only donor BMI but also weight loss history, among other donor characteristics, might be considered for optimal ASC-based therapy.
Keywords: adipose stromal/stem cells; bariatric surgery; inflammation; macrophages; obesity; weight loss.
Copyright © 2025 Amna Adnan et al. Stem Cells International published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Evaluation of the effect of donor weight on adipose stromal/stem cell characteristics by using weight-discordant monozygotic twin pairs.Stem Cell Res Ther. 2021 Sep 26;12(1):516. doi: 10.1186/s13287-021-02587-0. Stem Cell Res Ther. 2021. PMID: 34565451 Free PMC article.
-
Stromal-vascular fraction content and adipose stem cell behavior are altered in morbid obese and post bariatric surgery ex-obese women.Stem Cell Res Ther. 2015 Apr 14;6(1):72. doi: 10.1186/s13287-015-0029-x. Stem Cell Res Ther. 2015. PMID: 25884374 Free PMC article.
-
Functional Plasticity of Adipose-Derived Stromal Cells During Development of Obesity.Stem Cells Transl Med. 2016 Jul;5(7):893-900. doi: 10.5966/sctm.2015-0240. Epub 2016 May 13. Stem Cells Transl Med. 2016. PMID: 27177576 Free PMC article.
-
Influence of type 2 diabetes and obesity on adipose mesenchymal stem/stromal cell immunoregulation.Cell Tissue Res. 2023 Oct;394(1):33-53. doi: 10.1007/s00441-023-03801-6. Epub 2023 Jul 18. Cell Tissue Res. 2023. PMID: 37462786 Free PMC article. Review.
-
Message Transmission Between Adipocyte and Macrophage in Obesity.Adv Exp Med Biol. 2024;1460:273-295. doi: 10.1007/978-3-031-63657-8_9. Adv Exp Med Biol. 2024. PMID: 39287855 Review.
References
-
- Kurki A., Paakinaho K., Hannula M., et al. Promoting Cell Proliferation and Collagen Production With Ascorbic Acid 2-Phosphate-Releasing Poly(l-Lactide-Co-ε-Caprolactone) Membranes for Treating Pelvic Organ Prolapse. Regenerative Biomaterials . 2024;11 doi: 10.1093/rb/rbae060.rbae060 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

