Differential activation of monocytes and PMNs in orofacial granulomatosis patients induced by bacterial and non-bacterial stimuli
- PMID: 40396174
- PMCID: PMC12088978
- DOI: 10.3389/fimmu.2025.1522495
Differential activation of monocytes and PMNs in orofacial granulomatosis patients induced by bacterial and non-bacterial stimuli
Abstract
Introduction: Orofacial Granulomatosis (OFG) is a rare chronic inflammatory disorder characterized by persistent or recurrent swelling of the lips and oral mucosa, often accompanied by granulomatous inflammation in the orofacial region with limited effective treatment options available. Emerging evidence suggests an immune dysregulation in the development and progression of OFG. Immune cells, including monocytes and neutrophils (PMNs), are involved in autoimmune and inflammatory diseases by releasing pro-inflammatory and immunomodulatory molecules.
Methods: Considering that microbial agents have been suggested as potential triggers for OFG, in this study we evaluated the effect of LPS, fMLP and PMA on the activation of monocytes and PMNs purified by 11 OFG patients and 11 sex-and age-matched healthy donors (HDs).
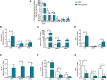
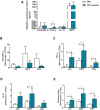
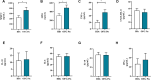
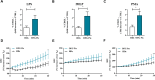
Results: Upon stimulation, OFG-derived monocytes displayed a higher release of pro-inflammatory cytokines (CXCL8/IL-8, IL-6, TNF-α, IL-33) compared to HDs. Conversely, OFG-derived monocytes showed a lower release of IL-10, IFN-γ compared to HDs. Upon stimulation, peripheral PMNs from OFG patients released large amounts of TNF-α and MPO compared to HDs. In addition, OFG-derived PMNs showed high percentages of activated PMNs (CD62L-) and increased ROS production compared to HDs. Compared to HDs, OFG patients presented higher serum levels of MMP-9, MPO and TNF-α, together with MPO-DNA complexes and citrullinated histone H3 (CitH3) (two biomarkers for neutrophil extracellular traps).
Discussion: These preliminary data suggest that in presence of various stimuli, monocytes and PMNs of OFG patients displayed an activated phenotype compared to HDs. Unraveling the interplay between bacterial triggers and immune cell function in OFG will be necessary to elucidate mechanisms driving this complex disease and identify novel therapeutic targets for improved management of OFG patients.
Keywords: NETs; ROS; bacterial stimuli; cytokines/chemokines; immunopathogenesis; inflammation.
Copyright © 2025 Palestra, Modestino, Ventrici, Monteforte, Memoli, Ferrara, Cristinziano, Poto, Rossi, Varricchi, De Paulis, Marone, Spadaro, Petraroli, Loffredo and Galdiero.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

