Microbiota-friendly diet ameliorates hypoalbuminemia in chronic kidney disease: evidence from NHANES
- PMID: 40396178
- PMCID: PMC12088941
- DOI: 10.3389/fimmu.2025.1546031
Microbiota-friendly diet ameliorates hypoalbuminemia in chronic kidney disease: evidence from NHANES
Abstract
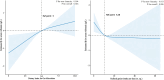
Chronic kidney disease (CKD) is a global health issue, affecting approximately 10% of the population. Hypoalbuminemia, a common complication in advanced CKD, is associated with poor prognosis. This study aimed to investigate the association between a microbiota-friendly dietary scoring system (Dietary Index for Gut Microbiota, DI-GM) and serum albumin levels in patients with CKD. We utilized a cross-sectional cohort from the NHANES 2007-2018, which included 2,947 CKD patients. Multivariable logistic regression and restricted cubic spline models were applied to analyze the relationship between DI-GM scores and serum albumin. Higher DI-GM scores were significantly associated with increased serum albumin levels (β = 0.18 g/L, 95% CI: 0.07-0.28, p = 0.002). Furthermore, each 1-point increase in DI-GM score was linked to a 15% reduction in the odds of hypoalbuminemia (OR: 0.85, 95% CI: 0.74-0.97, p = 0.014). The findings suggest that a high DI-GM diet may have beneficial effects in managing hypoalbuminemia in CKD patients by modulating gut microbiota composition and reducing inflammation. This diet pattern could be a promising dietary intervention for improving clinical outcomes in CKD patients, especially those at risk for malnutrition and inflammation.
Keywords: CKD; dietary index for gut microbiota; dietary pattern; hypoalbuminemia; serum albumin.
Copyright © 2025 Wang, Wen, Gao, Zhao and Miao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Association between dietary index for gut microbiota and chronic kidney disease: A cross-sectional study from U.S. population.Prev Med Rep. 2025 Apr 8;53:103060. doi: 10.1016/j.pmedr.2025.103060. eCollection 2025 May. Prev Med Rep. 2025. PMID: 40264750 Free PMC article.
-
Gastrointestinal symptoms, inflammation and hypoalbuminemia in chronic kidney disease patients: a cross-sectional study.BMC Nephrol. 2015 Dec 11;16:211. doi: 10.1186/s12882-015-0209-z. BMC Nephrol. 2015. PMID: 26651991 Free PMC article.
-
Dietary index for gut microbiota is associated with stroke among US adults.Food Funct. 2025 Feb 17;16(4):1458-1468. doi: 10.1039/d4fo04649h. Food Funct. 2025. PMID: 39898733
-
The Development and Evaluation of a Literature-Based Dietary Index for Gut Microbiota.Nutrients. 2024 Apr 3;16(7):1045. doi: 10.3390/nu16071045. Nutrients. 2024. PMID: 38613077 Free PMC article. Review.
-
Dietary Components That May Influence the Disturbed Gut Microbiota in Chronic Kidney Disease.Nutrients. 2019 Feb 27;11(3):496. doi: 10.3390/nu11030496. Nutrients. 2019. PMID: 30818761 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

