Cost-effectiveness and budget impact analysis of switching from apixaban to rivaroxaban treatment among patients with nonvalvular atrial fibrillation in a German healthcare setting
- PMID: 40396210
- PMCID: PMC12142402
- DOI: 10.57264/cer-2025-0008
Cost-effectiveness and budget impact analysis of switching from apixaban to rivaroxaban treatment among patients with nonvalvular atrial fibrillation in a German healthcare setting
Abstract
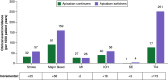
Aim: Direct oral anticoagulant (DOAC) switching often occurs in patients with nonvalvular atrial fibrillation (NVAF) for medical and nonmedical reasons. Limited data describe the economic consequences of DOAC switching in patients with NVAF. This study evaluates the cost-effectiveness and budget impact of initiating apixaban and switching to rivaroxaban versus initiating and continuing apixaban for patients with NVAF, from a German payer perspective. Materials & methods: Built on an existing model, a cohort-level lifetime Markov model was developed, including dynamic pricing assumptions to account for anticipated generic entry of DOACs. The modeled population (n = 1000) included German patients with NVAF, eligible for oral anticoagulation, who initiated on apixaban. The primary model outcome was the incremental cost-effectiveness ratio, assessed using cost per quality-adjusted life year (QALY) gained and a willingness-to-pay threshold of €48,750/QALY. A secondary model outcome was a 5-year budget impact analysis. Results: Switching patients from apixaban to rivaroxaban led to 285 additional events per 1000 patient years, resulting in 0.079 fewer QALYs and higher total costs per patient (€21,357 vs €16,390 for apixaban continuers). In the base case analysis (with generic pricing assumptions), switching from apixaban to rivaroxaban was dominated (i.e., less effective and more costly) by continuing apixaban. In the budget impact analysis (with generic pricing assumptions), switching from apixaban to rivaroxaban led to additional cumulative costs of €490 per patient over 5 years. Conclusion: Despite the introduction of generic discounting, switching patients with NVAF from apixaban to rivaroxaban led to higher total costs and fewer QALYs under base case assumptions, meaning apixaban switchers were dominated by apixaban continuers from a German payer perspective. Switching patients from apixaban to rivaroxaban also led to greater budget impact over 5 years.
Keywords: DOAC-to-DOAC switching; apixaban; cost–effectiveness; nonvalvular atrial fibrillation; rivaroxaban.
Conflict of interest statement
Competing interests disclosure
R Subash, E Dworatzek and A Kisser are employees and shareholders of Pfizer. M Zhang is an employee of Bristol Myers Squibb and the University of Southern California. M Hagan was an employee of Bristol Myers Squibb at the time of writing this study. T Strakosch was an employee of FIECON at the time of writing this study; FIECON received funding from the Pfizer/Bristol Myers Squibb Alliance in connection with the conduct of this study. C Salter, C Dickerson and E Stawowczyk are employees of Health Economics and Outcomes Research Ltd., HEOR Ltd. received funding from the Pfizer/Bristol Myers Squibb Alliance in connection with the development of this manuscript and conduct of the study. V Vasilopoulos was an employee of HEOR Ltd. at the time of writing this study. The authors have no other competing interests or relevant affiliations with any organization/entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
The authors have no other competing interests or relevant affiliations with any organization/entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures

Similar articles
-
Decision model to evaluate the cost of clinical events associated with switching from apixaban to rivaroxaban among patients with non-valvular atrial fibrillation in the United States and Germany.J Med Econ. 2025 Dec;28(1):224-234. doi: 10.1080/13696998.2025.2450933. Epub 2025 Feb 3. J Med Econ. 2025. PMID: 39819252
-
US cost-effectiveness analysis of apixaban compared with warfarin, dabigatran and rivaroxaban for nonvalvular atrial fibrillation, focusing on equal value of life years and health years in total.J Comp Eff Res. 2025 Jan;14(1):e240163. doi: 10.57264/cer-2024-0163. Epub 2024 Nov 28. J Comp Eff Res. 2025. PMID: 39606884 Free PMC article.
-
Cost-Effectiveness and Budget Impact Analysis of Apixaban and Rivaroxaban Versus Warfarin in the Prevention of Stroke in Patients With Non-Valvular Atrial Fibrillation (NVAF) in Iran.Clin Cardiol. 2024 Jun;47(6):e24311. doi: 10.1002/clc.24311. Clin Cardiol. 2024. PMID: 38923583 Free PMC article.
-
Latin American Clinical Epidemiology Network Series - Paper 2: Apixaban was cost-effective vs. acenocoumarol in patients with nonvalvular atrial fibrillation with moderate to severe risk of embolism in Chile.J Clin Epidemiol. 2017 Jun;86:75-83. doi: 10.1016/j.jclinepi.2016.05.018. Epub 2016 Oct 15. J Clin Epidemiol. 2017. PMID: 27756577 Review.
-
Direct comparative effectiveness and safety between non-vitamin K antagonist oral anticoagulants for stroke prevention in nonvalvular atrial fibrillation: a systematic review and meta-analysis of observational studies.Eur J Epidemiol. 2019 Feb;34(2):173-190. doi: 10.1007/s10654-018-0415-7. Epub 2018 Jun 8. Eur J Epidemiol. 2019. PMID: 29948370
References
-
- Di Carlo A, Bellino L, Consoli D et al. Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European Union: the FAI Project. Europace 21(10), 1468–1475 (2019). - PubMed
-
- Van Gelder IC, Rienstra M, Bunting KV et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): developed by the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC), with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur. Heart J. 45(36), 3314–3414 (2024). - PubMed
-
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22(8), 983–988 (1991). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
