Opioid Analgesia Following Pediatric Adenotonsillectomy: A Randomized Clinical Trial
- PMID: 40396501
- PMCID: PMC12312295
- DOI: 10.1002/ohn.1280
Opioid Analgesia Following Pediatric Adenotonsillectomy: A Randomized Clinical Trial
Abstract
Objective: To compare the safety and efficacy of nonopioid versus opioid pain management following adenotonsillectomy (AT) among pediatric patients.
Study design: An open-label randomized controlled trial.
Setting: Tertiary care children's hospital.
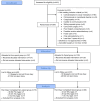
Methods: Patients aged 3 to 17 years undergoing AT were eligible. Participants were randomly assigned to receive either acetaminophen and ibuprofen (nonopioid group) or acetaminophen, ibuprofen, and oxycodone (opioid group). Pain scores and prevalence of emergency department (ED) visits, hospital readmission, and posttonsillectomy hemorrhage (PTH) were compared between groups.
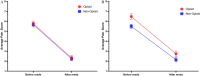
Results: From January 2019 to March 2020, 267 patients were enrolled and randomly assigned; 144 completed a postoperative pain diary. Of the 144, 69 (48%) patients received an opioid prescription, and 75 (52%) did not. Mean pain scores before (opioid: 5.78, 95% CI: 5.29-6.27 vs nonopioid: 5.66, 95% CI: 5.20-6.12) and after (opioid: 2.33, 95% CI: 1.89-2.78 vs nonopioid: 2.24, 95% CI: 1.82-2.66) analgesics were not significantly different between opioid and nonopioid groups. Although 7/75 (9%) from the nonopioid group crossed over and requested opioids, only 43/69 (62%) randomly assigned to receive opioid prescription consumed opioids. The rate of opioid consumption increased with increasing age: 18/71 (25%) patients aged 3 to 7 years, 22/57 (39%) 8 to 12 years, and 10/16 (63%) 13 to 17 years, P = .015. Differences in ED visits, hospital readmissions, and PTH between opioid and nonopioid groups were not significant.
Conclusion: Many children do not require opioid analgesics following AT, particularly children less than 8 years of age. Postoperative pain scores and outcomes were similar in opioid versus nonopioid groups. Opioid prescriptions should be limited or avoided altogether after pediatric AT.
Trial registration: Title: Nonopioids for analgesia after adenotonsillectomy in children; ID: NCT03618823, Clinicaltrials.gov.
Keywords: opioid prescription; pediatric opioid; postoperative opioid; postoperative pain; tonsillectomy pain.
© 2025 The Author(s). Otolaryngology–Head and Neck Surgery published by Wiley Periodicals LLC on behalf of American Academy of Otolaryngology–Head and Neck Surgery Foundation.
Conflict of interest statement
There are no conflicts of interest to disclose for any author.
Figures

Similar articles
-
The Association of Preoperative Hydrocodone and Acetaminophen With Total Opioid Requirement, and Pain Experience in Children Undergoing Adenotonsillectomy.Acta Anaesthesiol Scand. 2025 Sep;69(8):e70106. doi: 10.1111/aas.70106. Acta Anaesthesiol Scand. 2025. PMID: 40708157
-
Randomized clinical trial of non-opioid pain medications after intracapsular adenotonsillectomy.Int J Pediatr Otorhinolaryngol. 2025 Jun;193:112361. doi: 10.1016/j.ijporl.2025.112361. Epub 2025 Apr 21. Int J Pediatr Otorhinolaryngol. 2025. PMID: 40286466 Clinical Trial.
-
Tonsillectomy or adenotonsillectomy versus non-surgical management for obstructive sleep-disordered breathing in children.Cochrane Database Syst Rev. 2015 Oct 14;2015(10):CD011165. doi: 10.1002/14651858.CD011165.pub2. Cochrane Database Syst Rev. 2015. PMID: 26465274 Free PMC article.
-
Does Preoperative Pharmacogenomic Testing of Patients Undergoing TKA Improve Postoperative Pain? A Randomized Trial.Clin Orthop Relat Res. 2024 Feb 1;482(2):291-300. doi: 10.1097/CORR.0000000000002767. Epub 2023 Aug 18. Clin Orthop Relat Res. 2024. PMID: 37594401 Free PMC article. Clinical Trial.
-
As required versus fixed schedule analgesic administration for postoperative pain in children.Cochrane Database Syst Rev. 2015 Feb 26;2015(2):CD011404. doi: 10.1002/14651858.CD011404.pub2. Cochrane Database Syst Rev. 2015. PMID: 25719451 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

