Chirality-Induced Spin Selectivity at the Molecular Level: A Different Perspective to Understand and Exploit the Phenomenon
- PMID: 40396560
- PMCID: PMC12128100
- DOI: 10.1021/acs.jpclett.5c00755
Chirality-Induced Spin Selectivity at the Molecular Level: A Different Perspective to Understand and Exploit the Phenomenon
Abstract
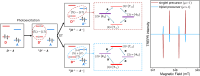
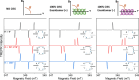
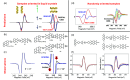
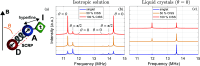
Investigating Chirality-Induced Spin Selectivity (CISS) at the molecular level offers a novel perspective, in between Chemistry and Physics, on this still not fully understood phenomenon. Indeed, the molecular approach offers an advantage point for understanding CISS by disentangling the role of chiral molecules from that of the surfaces. Here, we present an overview of experimental observations of CISS in electron transfer on isolated molecules in solution and the current status of theory to model the phenomenon. We discuss what is accomplished and which are the most important questions, and we propose experiments based on electron and nuclear magnetic resonance both to unravel open issues on the CISS effect in electron transfer and to apply it to quantum technologies.
Figures


Similar articles
-
Chirality-Induced Spin Selectivity in Composite Materials: A Device Perspective.Acc Chem Res. 2024 May 21;57(10):1478-1487. doi: 10.1021/acs.accounts.4c00077. Epub 2024 Apr 30. Acc Chem Res. 2024. PMID: 38687873 Free PMC article.
-
Chirality induced spin selectivity in electron transport investigated by scanning probe microscopy.J Phys Condens Matter. 2025 Jan 13;37(11). doi: 10.1088/1361-648X/ada478. J Phys Condens Matter. 2025. PMID: 39740349 Review.
-
Efficient Transfer of Chirality in Complex Hybrid Materials and Impact on Chirality-induced Spin Selectivity.Chem Mater. 2024 Nov 20;36(23):11449-11461. doi: 10.1021/acs.chemmater.4c02108. eCollection 2024 Dec 10. Chem Mater. 2024. PMID: 39678932 Free PMC article.
-
Chiral Induced Spin Selectivity.Chem Rev. 2024 Feb 28;124(4):1950-1991. doi: 10.1021/acs.chemrev.3c00661. Epub 2024 Feb 16. Chem Rev. 2024. PMID: 38364021 Free PMC article. Review.
-
The chirality-induced spin selectivity effect in asymmetric spin transport: from solution to device applications.Chem Sci. 2024 Oct 29;15(45):18751-18771. doi: 10.1039/d4sc05736h. eCollection 2024 Nov 20. Chem Sci. 2024. PMID: 39568626 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

