Synergistic treatment of linoleic acid and cefazolin on Staphylococcus aureus biofilm-related catheter infections
- PMID: 40396720
- PMCID: PMC12175515
- DOI: 10.1128/aem.00770-25
Synergistic treatment of linoleic acid and cefazolin on Staphylococcus aureus biofilm-related catheter infections
Abstract
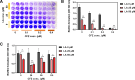
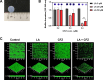
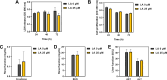
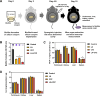
Staphylococcus aureus is a human pathogen that causes severe infections through biofilm formation. S. aureus biofilm is particularly susceptible to catheters in patients undergoing peritoneal dialysis. Although antibiotics are used to treat catheter infections, high-concentration treatments adversely affect human host immune systems and change the physicochemical properties of the catheters. To improve therapeutic outcomes without side effects, we combined antibiotics and natural products. In this study, we propose a combination of linoleic acid (LA) and cefazolin (CFZ) to treat S. aureus infections synergistically and apply it to catheter environments and in vivo systems. LA is a polyunsaturated fatty acid derived from natural products, and CFZ is a major antibiotic used to treat S. aureus catheter-related infections. The optimum synergistic condition was determined using silicon pad-forming biofilm similar to catheter materials. S. aureus biofilms were considerably inhibited in vitro and in vivo owing to the improved antibacterial effects. Furthermore, the combination negatively regulated the chemokine levels in the peritoneum, kidney, and liver extracted from mouse models. Moreover, it did not affect the cytotoxicity of human omentum mesothelial cells and the functions of the kidney and liver. Therefore, the combination of LA and CFZ could be a potential synergistic therapy for S. aureus catheter infections.IMPORTANCECatheter contamination is commonly caused by Staphylococcus aureus biofilm formation, primarily in peritoneal dialysis patients. Although antibiotics are used to treat catheter infections, high concentrations of antibiotics impair the immune system of the human host and alter the physicochemical properties of catheters. Therefore, it is crucial to improve therapeutic outcomes while minimizing the side effects of antibiotics. Combined treatments with natural products can be solutions to alleviate these problems. Our study offers a new synergistic combination (linoleic acid and cefazolin) for the control of catheter infections caused by S. aureus biofilms, especially in peritoneal dialysis.
Keywords: Staphylococcus aureus; catheter biofilm; cefazolin; linoleic acid; synergistic combination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Copper-coated carbon-infiltrated carbon nanotube surfaces effectively inhibit Staphylococcus aureus and Pseudomonas aeruginosa biofilm formation.Appl Environ Microbiol. 2025 Aug 20;91(8):e0105325. doi: 10.1128/aem.01053-25. Epub 2025 Jul 8. Appl Environ Microbiol. 2025. PMID: 40626868 Free PMC article.
-
The Effects of Low Concentrations of Nisin on Biofilm Formation by Staphylococcus aureus Isolated from Dairy Cattle.Pathogens. 2025 Jun 5;14(6):566. doi: 10.3390/pathogens14060566. Pathogens. 2025. PMID: 40559574 Free PMC article.
-
Antibacterial and antibiofilm potentials of vancomycin-loaded niosomal drug delivery system against methicillin-resistant Staphylococcus aureus (MRSA) infections.BMC Biotechnol. 2024 Jul 8;24(1):47. doi: 10.1186/s12896-024-00874-1. BMC Biotechnol. 2024. PMID: 38978013 Free PMC article.
-
Biofilm Formation in Dairy: A Food Safety Concern-Effect of biofilm production on antimicrobial susceptibility of Staphylococcus aureus bovine mastitis strains from the most prevalent Canadian spa types.J Dairy Sci. 2025 Aug;108(8):8176-8186. doi: 10.3168/jds.2024-25238. Epub 2024 Aug 8. J Dairy Sci. 2025. PMID: 39122151 Review.
-
Deciphering the dynamics of methicillin-resistant Staphylococcus aureus biofilm formation: from molecular signaling to nanotherapeutic advances.Cell Commun Signal. 2024 Mar 22;22(1):188. doi: 10.1186/s12964-024-01511-2. Cell Commun Signal. 2024. PMID: 38519959 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

