Linking oral microbiota to periodontitis and hypertension unveils that Filifactor alocis aggravates hypertension via infiltration of interferon-γ+ T cells
- PMID: 40396735
- PMCID: PMC12172497
- DOI: 10.1128/msystems.00084-25
Linking oral microbiota to periodontitis and hypertension unveils that Filifactor alocis aggravates hypertension via infiltration of interferon-γ+ T cells
Abstract
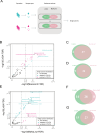
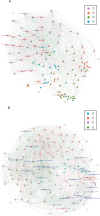
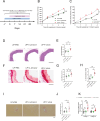
Periodontal disease (PD), an inflammatory disease initiated by oral microbiota, may aggravate hypertension (HTN). Few studies were employed to characterize the oral microbiota in hypertensive patients with periodontitis. To investigate the interplay between oral microbiota and hypertension in individuals with periodontitis, we initiated a metagenomic sequencing study on subgingival plaque and saliva samples sourced from HTN patients and those with hypertension and periodontitis (PDHTN). Our primary objective was to characterize species serving as pivotal links (bridge species) in exacerbating hypertension induced by periodontal disease. Within subgingival plaque and saliva specimens, we pinpointed 31 and 28 bridge species, respectively. Furthermore, we noted a decrease in the abundance of nitrate-reducing bacteria, such as Actinomyces spp., Rothia spp., and Veillonella spp., in PDHTN samples. Employing network analysis, we distinguished distinct polymicrobial clusters within the two patient groups. These bridge species coalesced into polymicrobial clusters, revealing intricate symbiotic and competitive relationships. To substantiate our findings, we leveraged an angiotensin II-infused animal model of ligature-induced periodontitis (LIP), confirming the contributory role of Filifactor alocis-a selectively analyzed subgingival bridge species-in exacerbating hypertension and upregulating the frequency of renal CD4+IFNγ+ and CD8+IFNγ+ T cells. Our study screened a list of species linking PD and HTN. PD may aggravate HTN by decreasing the abundance of nitrate-reducing bacteria and increasing the abundance of pathogens. Using an animal model, we demonstrated that F. alocis aggravates HTN via the accumulation of IFNγ+ T cells in the kidneys.
Importance: Both periodontal disease and hypertension are widely prevalent all over the world. PD may aggravate the development of HTN via oral microbiota. However, few studies were employed to characterize the oral microbiota in hypertensive patients with periodontitis. Here, the present study profiled the oral microbiota in hypertensive participants with periodontitis. We found that the depleted abundance of nitrate-reducing bacteria and the enriched abundance of pathogens. Finally, we validated the role of Filifactor alocis in exacerbating HTN via infiltration of IFNγ+ T cells in mice kidneys. Our study improved the understanding of oral microbiota linking PD and HTN.
Keywords: Filifactor alocis; IFNγ+ T cell; hypertension; oral microbiota; periodontitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Microbial Complexes in Subgingival Plaque: A Bacterial Meta-Taxonomic Study.J Clin Periodontol. 2025 Jul;52(7):983-998. doi: 10.1111/jcpe.14138. Epub 2025 Jun 4. J Clin Periodontol. 2025. PMID: 40467115
-
Bacterial translocation signatures and subgingival microbiome in individuals with periodontitis.Clin Oral Investig. 2025 May 7;29(6):288. doi: 10.1007/s00784-025-06363-9. Clin Oral Investig. 2025. PMID: 40329006
-
Oral Microbial Dysbiosis Driven by Periodontitis Facilitates Oral Squamous Cell Carcinoma Progression.Cancers (Basel). 2025 Jun 28;17(13):2181. doi: 10.3390/cancers17132181. Cancers (Basel). 2025. PMID: 40647479 Free PMC article.
-
Is there a link between the abundance of nitrate-reducing bacteria and arterial hypertension? A systematic review.Nitric Oxide. 2025 Aug;157:19-33. doi: 10.1016/j.niox.2025.04.001. Epub 2025 Apr 11. Nitric Oxide. 2025. PMID: 40220988
-
Advancing periodontitis microbiome research: integrating design, analysis, and technology.Front Cell Infect Microbiol. 2025 Jun 23;15:1616250. doi: 10.3389/fcimb.2025.1616250. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40625831 Free PMC article. Review.
References
-
- Joffres M, Falaschetti E, Gillespie C, Robitaille C, Loustalot F, Poulter N, McAlister FA, Johansen H, Baclic O, Campbell N. 2013. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: a cross-sectional study. BMJ Open 3:e003423. doi: 10.1136/bmjopen-2013-003423 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 82330015,81991503,81991500/National Natural Science Foundation of China
- 2023YFA1801100,2023YFA1801104/National Key Research and Development Program of China
- BX20230226/China National Postdoctoral Program for Innovative Talents
- SHSMU-ZDCX20212500/Innovative Research Team of High-level Local University in Shanghai (Innovative Research Team of High-level Local Universities in Shanghai)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

