Unraveling the cross-talk between a highly virulent PEDV strain and the host via single-cell transcriptomic analysis
- PMID: 40396761
- PMCID: PMC12172440
- DOI: 10.1128/jvi.00555-25
Unraveling the cross-talk between a highly virulent PEDV strain and the host via single-cell transcriptomic analysis
Abstract
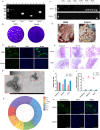
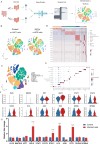
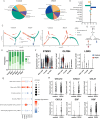
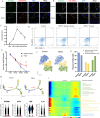
Porcine epidemic diarrhea virus (PEDV) causes severe intestinal damage and high mortality in neonatal piglets. The continuous emergence of new strains has brought new challenges to prevention and control. In this study, we isolated and characterized a prevalent PEDV virulent strain and analyzed 19,612 jejunal cells from PEDV-infected and control piglets using single-cell sequencing, revealing significant changes in cellular composition, gene expression, and intercellular communication. In response to PEDV infection, epithelial repair was enhanced through increased proliferation and differentiation of stem cells, transit-amplifying (TA) cells, and intestinal progenitor cells into enterocytes. Additionally, PEDV disrupted intercellular communication, compromising epithelial functionality while triggering immune responses, with IFN-γ and IL-10 signaling activation acting as critical regulators of immune balance and tissue homeostasis. Beyond enterocytes, viral genes were detected in various other cell types. Further experiments confirmed that PEDV could initiate replication in B and T lymphocytes but was unable to produce infectious progeny, with T cells additionally undergoing virus-induced apoptosis. These findings provide new insights into PEDV tropism, immune evasion, and epithelial repair, revealing complex host-pathogen interactions that shape disease progression and tissue regeneration, thereby contributing to a better understanding of enteric coronavirus pathogenesis.IMPORTANCEThe persistent circulation of porcine epidemic diarrhea virus (PEDV) poses a major threat to the swine industry, with emerging strains complicating prevention and control efforts. Currently, no effective measures completely prevent virus transmission, highlighting the need to understand PEDV-host interactions. In this study, we isolated a prevalent virulent strain and used single-cell sequencing to identify new PEDV-infected cell types and explore the complex interplay between the host and PEDV. These findings provide essential insights into viral pathogenesis and facilitate the design of targeted antiviral interventions.
Keywords: PEDV; host-pathogen interactions; intestinal regeneration; lymphocyte infection; single-cell RNA-seq.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Engineering a recombination-resistant live attenuated vaccine candidate with suppressed interferon antagonists for PEDV.J Virol. 2025 Jul 22;99(7):e0045125. doi: 10.1128/jvi.00451-25. Epub 2025 Jun 12. J Virol. 2025. PMID: 40503881 Free PMC article.
-
IFITM proteins are key entry factors for porcine epidemic diarrhea coronavirus.J Virol. 2025 Jun 17;99(6):e0202824. doi: 10.1128/jvi.02028-24. Epub 2025 May 12. J Virol. 2025. PMID: 40353666 Free PMC article.
-
The role of innate immune responses against two strains of PEDV (S INDEL and non-S INDEL) in newborn and weaned piglets inoculated by combined orogastric and intranasal routes.Front Immunol. 2025 Jun 16;16:1584785. doi: 10.3389/fimmu.2025.1584785. eCollection 2025. Front Immunol. 2025. PMID: 40589734 Free PMC article.
-
The biomechanical phenomena observed in the cell invasion pathway of porcine epidemic diarrhea virus: a review.Arch Virol. 2025 May 26;170(7):139. doi: 10.1007/s00705-025-06326-1. Arch Virol. 2025. PMID: 40418401 Review.
-
Traditional Chinese medicine as a promising choice for future control of PEDV.Virus Res. 2025 Jun;356:199572. doi: 10.1016/j.virusres.2025.199572. Epub 2025 Apr 10. Virus Res. 2025. PMID: 40220931 Review.
References
-
- Jung K, Eyerly B, Annamalai T, Lu Z, Saif LJ. 2015. Structural alteration of tight and adherens junctions in villous and crypt epithelium of the small and large intestine of conventional nursing piglets infected with porcine epidemic diarrhea virus. Vet Microbiol 177:373–378. doi: 10.1016/j.vetmic.2015.03.022 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

