Plasmid diversity of Serratia marcescens and Klebsiella pneumoniae isolates involved in two carbapenem-resistant Enterobacteriaceae outbreaks in a Swiss hospital
- PMID: 40396774
- PMCID: PMC12210979
- DOI: 10.1128/spectrum.03284-24
Plasmid diversity of Serratia marcescens and Klebsiella pneumoniae isolates involved in two carbapenem-resistant Enterobacteriaceae outbreaks in a Swiss hospital
Abstract
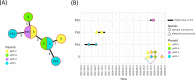
This study investigates two distinct carbapenemase-producing Enterobacteriaceae outbreaks involving patients and contaminated sink traps at the University Hospital of Lausanne. It focuses on the diversity and transmission dynamics of plasmids carrying carbapenemase genes. Between 2022 and 2023, 57 carbapenem-resistant Klebsiella pneumoniae and Serratia marcescens isolates were collected and analyzed. Core-genome MLST confirmed genetic similarity among isolates, linking the outbreaks to sink trap contamination. DNA extraction, sequencing (MinION/Illumina MiSeq), and assembly were performed, followed by ARG screening and plasmid typing. Plasmids were annotated, clustered, and compared using core SNP distances and structural analyses. Known plasmids were identified through PLSDB database matching. Eight MLST types were identified in K. pneumoniae and one (ST356) in S. marcescens. Analysis of 52 bla-carrying plasmids revealed 22 plasmid clusters, including 6 blaNDM-1 clusters in K. pneumoniae and 4 blaKPC-2 clusters in S. marcescens. Plasmids showed close relatedness within and across patient and environmental isolates, with core SNP distances ranging from 0 to 18. Some blaNDM-1 plasmids in K. pneumoniae clustered tightly, suggesting persistence and potential cross-contamination routes. The findings highlight sink traps as critical reservoirs for carbapenem-resistant Enterobacteriaceae and plasmids, promoting resistance gene spread across species. The observed plasmid diversity indicates transmission can occur independently of bacterial clonal spread, challenging traditional outbreak definitions.
Importance: This research is critical in addressing the growing threat of antibiotic resistance, driven by the spread of resistance genes through plasmids. Plasmids, which can transfer between different bacteria, play a major role in spreading multidrug resistance, posing a serious challenge to healthcare systems worldwide. By highlighting how plasmids can move independently of bacterial spread, this study reveals the complexity of resistance transmission. It also underscores the importance of environmental reservoirs, such as hospital sink traps, in harboring and spreading resistant bacteria. These findings emphasize the need for better monitoring of plasmids and targeted infection control measures to prevent the spread of resistance genes and protect the effectiveness of current antibiotics.
Keywords: Enterobacteriaceae; carbapenemase; outbreak; plasmids; typing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Otter JA, Burgess P, Davies F, Mookerjee S, Singleton J, Gilchrist M, Parsons D, Brannigan ET, Robotham J, Holmes AH. 2017. Counting the cost of an outbreak of carbapenemase-producing Enterobacteriaceae: an economic evaluation from a hospital perspective. Clin Microbiol Infect 23:188–196. doi: 10.1016/j.cmi.2016.10.005 - DOI - PubMed
-
- Hassoun-Kheir N, Hussien K, Karram M, Saffuri M, Badaan S, Peleg S, Aboelhega W, Warman S, Alon T, Pollak D, Szwarcwort Cohen M, Paul M. 2023. Clinical significance and burden of carbapenem-resistant Enterobacterales (CRE) colonization acquisition in hospitalized patients. Antimicrob Resist Infect Control 12:129. doi: 10.1186/s13756-023-01323-y - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

