Methods for Controlling Small GTPase Activity
- PMID: 40396811
- PMCID: PMC12247031
- DOI: 10.1002/cbic.202500156
Methods for Controlling Small GTPase Activity
Abstract
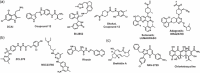
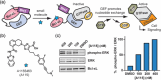
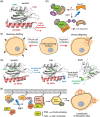
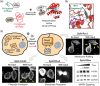
Small GTPases comprise a diverse class of signaling proteins in mammalian cells and regulate a variety of cellular processes such as cell growth, cell movement, vesicle formation, and nuclear transport. Due to their involvement in critical cellular pathways, changes in the activation state of small GTPases due to genetic mutations or alterations in gene expression can lead to human diseases. As such, the ability to control the activity of small GTPases is paramount in understanding the precise role these proteins play in human biology and in reducing their impacts on related diseases. Herein, important advances made in the development of small-molecule- and protein engineering-based strategies to control the activity of small GTPases are presented. Current approaches within each area are discussed within their historical contexts along with commentary on the importance that each technology has had on improving the ability to regulate small GTPase activity. Given this ever-evolving toolbox for controlling small GTPase signaling, continued growth in the study of this protein class is anticipated.
Keywords: inhibitors; protein engineering; signal transductions; small GTPases; split proteins.
© 2025 The Author(s). ChemBioChem published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Relationship between Pituitary Gland and Stem Cell in the Aspect of Hormone Production and Disease Prevention: A Narrative Review.Endocr Metab Immune Disord Drug Targets. 2025;25(7):509-526. doi: 10.2174/0118715303314551241031093717. Endocr Metab Immune Disord Drug Targets. 2025. PMID: 39812047 Review.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

