A conserved role for centriolar satellites in translation of centrosomal and ciliary proteins
- PMID: 40396915
- PMCID: PMC12094185
- DOI: 10.1083/jcb.202408042
A conserved role for centriolar satellites in translation of centrosomal and ciliary proteins
Abstract
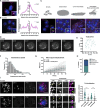
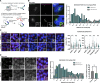
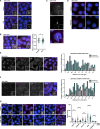
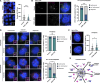
Centriolar satellites are cytoplasmic particles found in the vicinity of centrosomes and cilia whose specific functional contribution has long been unclear. Here, we identify Combover as the Drosophila ortholog of the main scaffolding component of satellites, PCM1. Like PCM1, Combover localizes to cytoplasmic foci containing centrosomal proteins and its depletion or mutation results in centrosomal and ciliary phenotypes. Strikingly, however, the concentration of satellites near centrosomes and cilia is not a conserved feature, nor do Combover foci display directed movement. Proximity interaction analysis revealed not only centrosomal and ciliary proteins, but also RNA-binding proteins and proteins involved in quality control. Further work in Drosophila and vertebrate cells found satellites to be associated with centrosomal and ciliary mRNAs, as well as evidence for protein synthesis occurring directly at satellites. Given that PCM1 depletion does not affect overall protein levels, we propose that satellites instead promote the coordinate synthesis of centrosomal and ciliary proteins, thereby facilitating the formation of protein complexes.
© 2025 Pachinger et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures







Similar articles
-
A subset of evolutionarily conserved centriolar satellite core components is crucial for sperm flagellum biogenesis.Theranostics. 2025 Jun 12;15(14):7025-7044. doi: 10.7150/thno.117118. eCollection 2025. Theranostics. 2025. PMID: 40585997 Free PMC article.
-
The Unkempt RNA-binding protein reveals a local translation program in centriole overduplication.J Cell Biol. 2025 Aug 4;224(8):e202407196. doi: 10.1083/jcb.202407196. Epub 2025 Jul 23. J Cell Biol. 2025. PMID: 40699149
-
CCHCR1 links P-body proteins to the centrosome and is required for ciliogenesis through interacting with OFD1 and PCM1.Cell Mol Biol Lett. 2025 Aug 29;30(1):103. doi: 10.1186/s11658-025-00780-0. Cell Mol Biol Lett. 2025. PMID: 40883668 Free PMC article.
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
Cited by
-
The C. elegans homolog of Sjögren's Syndrome Nuclear Antigen 1 is required for the structural integrity of the centriole and bipolar mitotic spindle assembly.bioRxiv [Preprint]. 2024 Oct 4:2024.10.03.616528. doi: 10.1101/2024.10.03.616528. bioRxiv. 2024. Update in: Nat Commun. 2025 Jun 5;16(1):5220. doi: 10.1038/s41467-025-59939-0. PMID: 39803516 Free PMC article. Updated. Preprint.
-
C. elegans SSNA-1 is required for the structural integrity of centrioles and bipolar spindle assembly.Nat Commun. 2025 Jun 5;16(1):5220. doi: 10.1038/s41467-025-59939-0. Nat Commun. 2025. PMID: 40473601 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

