Activated Partial Thromboplastin Time and Anti-IIa Monitoring in Argatroban Anticoagulation in COVID-19 Patients on Venovenous Extracorporeal Membrane Oxygenation
- PMID: 40396972
- PMCID: PMC12099084
- DOI: 10.1177/10760296251341315
Activated Partial Thromboplastin Time and Anti-IIa Monitoring in Argatroban Anticoagulation in COVID-19 Patients on Venovenous Extracorporeal Membrane Oxygenation
Abstract
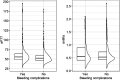
Unfractionated heparin has long been considered the standard anticoagulation in ECMO, despite some pitfalls such as heparin resistance, heparin induced thrombocytopenia (HIT), etc Recently, some centres started to increasingly use argatroban for this purpose, typically using activated partial thromboplastin time (aPTT) for its monitoring. Direct monitoring of the efficacy of argatroban using Anti-IIa is not yet an established method, although it might be more appropriate as it targets the same pathway.An observational study was performed in adult veno-venous ECMO patients hospitalized with SARS-CoV-2 infection anticoagulated with argatroban to an aPTT target of 40-60 s and Anti-IIa target of 0.4-0.6 µg/mL. Bleeding and thrombotic complications were monitored.Forty-four VV ECMO patients were included, with an overall hospital mortality of approx. 50%. No life-threatening thrombotic events were recorded. The risk of bleeding complications significantly increased with aPTT above 52.7 s and with Anti-IIa values over 0.78 µg/mL. Using the above cut-offs for both the aPTT and Anti-IIa and their combination, the negative predictive value for bleeding was approximately 90%.It seems that the generally recommended limits for Anti-IIa of 1.5 µg/mL may be high. However, further data are needed to confirm lower limits.Trial Registration:retrospectively registered in ClinicalTrials.gov, NCT06038682.
Keywords: Anti-IIa; activated partial thromboplastin time (aPTT); anticoagulation; argatroban; extracorporeal oxygenation (ECMO); monitoring.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
A safety comparison of heparin and argatroban anticoagulation in veno-venous extracorporeal membrane oxygenation with a focus on bleeding.Transfus Med. 2025 Feb;35(1):75-81. doi: 10.1111/tme.13102. Epub 2024 Oct 7. Transfus Med. 2025. PMID: 39375884 Free PMC article.
-
Argatroban versus heparin in patients without heparin-induced thrombocytopenia during venovenous extracorporeal membrane oxygenation: a propensity-score matched study.Crit Care. 2021 Apr 29;25(1):160. doi: 10.1186/s13054-021-03581-x. Crit Care. 2021. PMID: 33910609 Free PMC article.
-
Anticoagulation with argatroban using hemoclot™ targets is safe and effective in CARDS patients receiving venovenous extracorporeal membrane oxygenation: An exploratory bi-centric cohort study.Thromb Res. 2024 Apr;236:161-166. doi: 10.1016/j.thromres.2024.02.026. Epub 2024 Mar 2. Thromb Res. 2024. PMID: 38452448
-
Argatroban Anticoagulation for Adult Extracorporeal Membrane Oxygenation: A Systematic Review.J Intensive Care Med. 2022 Apr;37(4):459-471. doi: 10.1177/0885066621993739. Epub 2021 Mar 3. J Intensive Care Med. 2022. PMID: 33653194
-
Reducing harm associated with anticoagulation: practical considerations of argatroban therapy in heparin-induced thrombocytopenia.Drug Saf. 2009;32(3):203-18. doi: 10.2165/00002018-200932030-00003. Drug Saf. 2009. PMID: 19338378 Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

