DNA Origami-Based CD44-Targeted Therapy Silences Stat3 Enhances Cartilage Regeneration and Alleviates Osteoarthritis Progression
- PMID: 40396977
- PMCID: PMC12362795
- DOI: 10.1002/advs.202503939
DNA Origami-Based CD44-Targeted Therapy Silences Stat3 Enhances Cartilage Regeneration and Alleviates Osteoarthritis Progression
Abstract
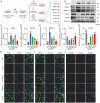
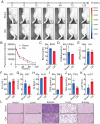
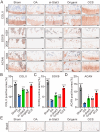
Osteoarthritis (OA) is a widespread musculoskeletal disorder affecting ≈600 million people globally, and small interfering RNA (siRNA) therapy shows potential in targeting OA progression. However, the efficient and targeted delivery of siRNA remains a major challenge due to issues with tissue specificity and degradation in vivo. In this study, A DNA origami-based chondrocyte-targeted delivery system (OCS) is designed for siRNA delivery to OA-affected cartilage. The DNA origami is engineered to load with siRNA targeting signal transducer and activator of transcription 3 (Stat3), a key regulator of inflammation and cartilage degradation, and is functionalized with anti-CD44 aptamers for selective targeting of OA chondrocytes. In vitro, the DNA origami system effectively delivers siRNA to diseased chondrocytes, silencing matrix metalloproteinases expression and reducing inflammation. In OA rat models, it preserves cartilage integrity, promotes regeneration, and mitigates ECM degradation without evident side effects. These findings highlight DNA origami as a promising platform for siRNA-based OA therapy, offering a promising solution to the challenges of targeted and efficient siRNA delivery.
Keywords: CD44 targeting; DNA triangle origami; osteoarthritis; si‐Stat3.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
