Plasma chemokines indicate enhanced bleeding in patients with chronic coronary syndrome undergoing percutaneous coronary stenting
- PMID: 40397144
- PMCID: PMC12283909
- DOI: 10.1007/s00392-025-02675-8
Plasma chemokines indicate enhanced bleeding in patients with chronic coronary syndrome undergoing percutaneous coronary stenting
Abstract
Background: Patients with coronary artery disease (CAD) are at increased risk of developing ischemic events and contemporary antiplatelet therapy often leads to bleeding events following percutaneous coronary intervention (PCI). Glycoprotein VI (GPVI) is the key receptor of collagen-dependent thrombus formation and crucial for platelet homeostasis.
Methods: We analysed the influence of GPVI inhibition with revacept in a randomized double-blinded trial enrolling 334 patients with CAD undergoing elective PCI. Ex vivo platelet function analyses were assessed alongside plasma chemokine concentrations. We then elucidate changes of GPVI-dependent chemokine concentrations in patients with bleeding events during the 30-day clinical follow-up.
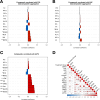
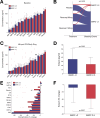
Results: Changes in platelet function occur in patients with revacept treatment and are associated with a characteristic alteration of circulating chemokine concentrations. Further, patients with adverse bleeding events share a distinct fingerprint of chemokines that is associated with modulation of in vitro platelet functions. In addition, assessment of GPVI-associated changes in chemokine signalling and platelet functions demonstrated an increased diagnostic value in patients with CAD and might improve early risk discrimination for bleeding events.
Conclusion: The composition of platelet-derived chemokines correlated with platelet functions following antiplatelet treatment. Thus, assessment of chemokines may offer the perspective to identify patients at increased risk for bleeding events. Likewise, modulation of platelet chemokines in patients with revacept treatment contributes to the efficacy of antiplatelet treatment and might attenuate pathophysiological cascades leading to haemorrhagic diathesis in patients with CAD.
Keywords: Antiplatelet treatment; Bleeding; Chemokine signalling; Coronary artery disease; Soluble glycoprotein VI.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no conflict of interest related to this work. The initial study (Intracoronary Stenting and Antithrombotic Regimen: Lesion Platelet Adhesion as Selective Target of Endovenous Revacept in Patients With Chronic Coronary Syndromes Undergoing Percutaneous Coronary Intervention) was funded by the German Center for Cardiovascular Research (DZHK), Deutsches Herzzentrum München, Federal Ministry of Education and Research (BMBF), and advanceCOR GmbH (the manufacturer of Revacept, patent PCT/EP2018/067952). Tobias Harm receives research funding from the German Cardiac Society (DGK) Clinical Scientist Program. Götz Münch and Meinrad Paul Gawaz are co-founders of advanceCOR GmbH. Kristin Adler is an employee of advanceCOR GmbH. No other disclosures were reported.
Figures


References
-
- Reny JL, Fontana P, Hochholzer W, Neumann FJ, Ten Berg J, Janssen PW, Geisler T, Gawaz M, Marcucci R, Gori AM, Cuisset T, Alessi MC, Berdague P, Gurbel PA, Yong G, Angiolillo DJ, Aradi D, Beigel R, Campo G, Combescure C (2016) Vascular risk levels affect the predictive value of platelet reactivity for the occurrence of MACE in patients on clopidogrel. Systematic review and meta-analysis of individual patient data. Thromb Haemost 115(4):844–855. 10.1160/th15-09-0742 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

