Molecular mechanisms of arecoline-induced oral cancer: a network toxicology and molecular docking techniques integrated analysis
- PMID: 40397259
- PMCID: PMC12095829
- DOI: 10.1007/s12672-025-02659-0
Molecular mechanisms of arecoline-induced oral cancer: a network toxicology and molecular docking techniques integrated analysis
Abstract
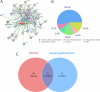
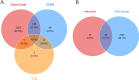
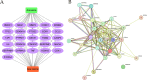
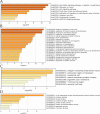
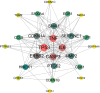
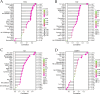
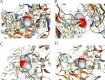
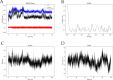
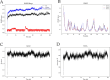
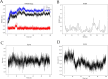
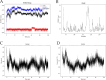
The IARC classified betel nut as Group 1 carcinogen (2004) and arecoline as Group 2B carcinogen (2020), with approximately one-third of global oral cancer cases attributed to smokeless tobacco or betel nut consumption. While current evidence establishes an association between arecoline and oral cancer, the underlying molecular mechanisms remain complex and poorly elucidated. This study employs network toxicology integrated with molecular docking techniques to systematically investigate the potential molecular pathogenesis of arecoline-induced oral cancer, aiming to provide novel insights for targeted therapeutic strategies. The SMILES structure of arecoline was retrieved from PubChem for foundational data preparation. Toxicity profiling was conducted using ProTox-3.0 and ADMETlab databases. Potential targets of arecoline were identified via STITCH and SwissTargetPrediction. Oral cancer-related targets were collated from GeneCards, OMIM, and TTD. Intersection analysis between arecoline targets and oral cancer-associated targets was performed to identify shared targets, which were further utilized to construct compound-target regulatory network and subjected to PPI, GO, and KEGG analyses. Core targets driving oral cancer were screened using the cytoHubba plugin. Then, the correlation between core targets and immune cell infiltration in oral cancer was explored, and molecular docking validated the binding affinity of arecoline to core targets. Finally, Gromacs 2022.3 software was used to simulate the molecular dynamics of the complexes obtained by molecular docking for 100 ns. Using the STITCH and SwissTargetPrediction databases, a total of 46 potential targets of arecoline were identified. Concurrently, 2,375 oral cancer-related targets were retrieved from GeneCards, OMIM, and TTD. Intersection analysis of these two target sets yielded 26 overlapping targets. PPI analysis revealed that TP53, IL6, SNAI1, and CASP3 occupied central positions in the network, exhibiting extensive interactions with other target proteins. Enrichment analysis comprehensively elucidated the molecular functions, biological processes, cellular components, and associated pathways of these overlapping targets. Further screening using Cytoscape software identified four core targets: TP53, TNF, IL6, and CASP3. Immune infiltration analysis indicated that the expression levels of TP53, TNF, IL6, and CASP3 in oral cancer tissues were positively correlated with the infiltration levels of immune cells, including CD8 + T cells, Th1 cells, NK cells, and macrophages. Molecular docking experiments demonstrated strong binding activities between arecoline and TP53, IL6, and CASP3, while TNF also exhibited moderate binding affinity. Dynamic simulation further verified the stable binding of arecoline to TP53, TNF, IL6 and CASP3. Arecoline may induce oral cancer by acting on core targets including TP53, TNF, IL6, and CASP3, which interfere with normal cellular growth regulation, inflammatory responses, and apoptotic mechanisms. Therapeutic strategies targeting TP53, TNF, IL6, and CASP3 may represent novel research directions for clinical diagnosis and treatment of oral cancer.
Keywords: Arecoline; CASP3; IL6; Network toxicology; Oral cancer; TNF; TP53.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Kozlakidis Z, Cheong I, Wang H. Betel nut and arecoline: past, present, and future trends. Innov Digit Health Diagn Biomarkers. 2022;2022(2):64–72.
-
- Liu YJ, Peng W, Hu MB, Xu M, Wu CJ. The pharmacology, toxicology and potential applications of arecoline: a review. Pharm Biol. 2016;54(11):2753–60. - PubMed
-
- Myers AL. Metabolism of the areca alkaloids - toxic and psychoactive constituents of the areca (betel) nut. Drug Metab Rev. 2022;54(4):343–60. - PubMed
-
- Kuo TM, Luo SY, Chiang SL, Yeh KT, Hsu HT, Wu CT, Lu CY, Tsai MH, Chang JG, Ko YC. Fibrotic effects of arecoline N-oxide in oral potentially malignant disorders. J Agric Food Chem. 2015;63(24):5787–94. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
