Olaparib Monotherapy or in Combination with Abiraterone for the Treatment of Patients with Metastatic Castration-Resistant Prostate Cancer (mCRPC) and a BRCA Mutation
- PMID: 40397306
- PMCID: PMC12106333
- DOI: 10.1007/s11523-025-01146-4
Olaparib Monotherapy or in Combination with Abiraterone for the Treatment of Patients with Metastatic Castration-Resistant Prostate Cancer (mCRPC) and a BRCA Mutation
Abstract
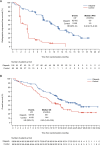
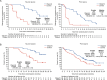
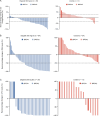
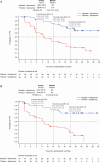
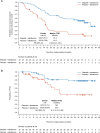
Treatment strategies to improve outcomes in patients with metastatic castration-resistant prostate cancer (mCRPC) are evolving. Of particular interest are therapies that target DNA damage responses in tumor cells by inhibiting poly(ADP-ribose) polymerase (PARP) activity. Several PARP inhibitors have recently received regulatory approval for the treatment of patients with mCRPC, of which olaparib was the first for prostate cancer. Olaparib received approval as a monotherapy following the PROfound study (NCT02987543) and in combination with abiraterone following the PROpel study (NCT03732820) for mCRPC. Both PROfound (homologous recombination repair mutation biomarker-selected) and PROpel (biomarker unselected) patients demonstrated statistically significant longer radiographic progression-free survival (rPFS) with olaparib versus their respective control arms in the intention-to-treat population. In both studies, the greatest clinical benefit with olaparib was seen in patients with BRCA1 and/or BRCA2 mutations (BRCAm): PROfound rPFS hazard ratio (HR) 0.22 (95% confidence interval [CI] 0.15-0.32); PROpel rPFS HR 0.23 (95% CI 0.12-0.43). Clinical benefit was also observed in terms of overall survival: PROfound HR 0.63 (95% CI 0.42-0.95); PROpel HR 0.29 (95% CI 0.14-0.56). We provide a comprehensive overview of the utility of olaparib for patients with mCRPC harboring a BRCAm. Key clinical and safety data in BRCAm subgroup populations are discussed, predominantly based on findings from PROfound and PROpel, as well as investigator-initiated studies, to help inform treatment decision-making in this patient population. We also discuss the importance of genetic testing to identify patients who may optimally benefit from treatment with olaparib, either as a monotherapy or in combination with abiraterone.
Plain language summary
In the USA, prostate cancer is the most commonly diagnosed cancer in men. It affects approximately one in eight men during their lifetime. Metastatic castration-resistant prostate cancer (mCRPC) occurs when the cancer spreads beyond the prostate gland and the disease progresses despite treatment with standard hormonal therapy. Patients who have cancers with mutations in BRCA1 and/or BRCA2 genes have poor outcomes, and additional life-prolonging treatments are needed. Olaparib is a drug approved to treat certain patients with mCRPC, both alone and in combination with abiraterone. Approval was based on two landmark clinical trials called PROfound and PROpel. PROfound compared olaparib directly with the hormonal therapies abiraterone or enzalutamide. PROpel evaluated whether combining olaparib with abiraterone would delay the progression of cancer compared with just abiraterone. After these two studies were completed, results were analyzed specifically in patients who had a BRCA mutation in their cancer. We have compiled the results in patients with mCRPC with BRCA mutations and show that both olaparib on its own or in combination with abiraterone resulted in substantial clinical benefits in delaying disease progression and improving survival over standard treatments for mCRPC. The side effects that patients with a BRCA mutation experienced were similar to those in the overall patient population originally analyzed. We also discuss the importance of testing men with prostate cancer for these genetic mutations before starting treatment to help identify patients who may benefit the most from olaparib on its own or in combination with abiraterone.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: Financial support was provided by AstraZeneca and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, who are codeveloping olaparib. Conflict of interest: Fred Saad has received consultancy and advisory fees from AbbVie, Advanced Accelerator Applications, Astellas Pharma, AstraZeneca/MedImmune, Bayer, Janssen Oncology, Knight Therapeutics, Myovant Sciences, Novartis, Pfizer, and Sanofi; honoraria from AbbVie, Advanced Accelerator Applications, Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb, Janssen Oncology, Knight Therapeutics, Merck, Myovant Sciences, Novartis, Pfizer, and Sanofi; and institutional research funding from Advanced Accelerator Applications, Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb, Janssen Oncology, Merck, Novartis, Pfizer, and Sanofi. Andrew J. Armstrong has received consultancy or advisory fees from Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Clovis Oncology, Dendreon, Exelixis, FORMA Therapeutics, GoodRX, Janssen, Merck, Myovant Sciences, Novartis, and Pfizer; travel and accommodation expenses from Astellas; and institutional research funding from Amgen, Astellas Pharma, AstraZeneca, Bayer, BeiGene, Bristol Myers Squibb, Constellation Pharmaceuticals, Dendreon, FORMA Therapeutics, Janssen Oncology, Gilead Sciences, Merck, Novartis, Pfizer, and Roche/Genentech and holds institutional patents, royalties, and other intellectual property for circulating tumor cell novel capture technology. Neal Shore has acted in a consulting or advisory role for AbbVie, Accord, Alessa Therapeutics, Amgen, Antev, Asieris, Astellas, AstraZeneca, Aura Biosciences, Bayer, Bioprotect, Bristol Myers Squibb, Boston Scientific, CGOncology, Clarity, Dendreon, Exact Imaging, Ferring, Fize Medical, Foundation Medicine, Invitae, Janssen, Lantheus, Lilly, Dxhealth, Merck, Minomic, Myriad, Novartis, Pfizer, Photocure, Promaxo, Protara, Sanofi Genzyme, Telix, Tolmar, and Urogen; and in a leadership or fiduciary role for Alessa Photocure and Tuteix. Neal Shore is an editorial board member of Targeted Oncology and as such was not involved in the selection of peer reviewers for the manuscript or any of the subsequent editorial decisions. Daniel J. George has received grants from Calithera, Convergence, and Corvus; grants and personal fees from Astellas, Bristol Myers Squibb, Johnson and Johnson Pharmaceuticals, Merck Sharp & Dohme, Novartis, and Pfizer; personal fees from AstraZeneca, Axess Oncology, Capio Bioscience, Flatiron, Michael J Hennessey Associates, Millennium Medical Publishing, Myovant Sciences Inc, NCI Genitourinary, Nektar Therapeutics, Physician Education Resource, UroGPO, Vizuri Health Sciences, Platform Q, Propella Therapeutics, RevHealth, Seattle Genetics, WebMD, and Xcures; grants, personal fees, and non-financial support from Bayer Healthcare Pharmaceuticals, and Exelixis Inc; personal fees and non-financial support from UroToday; and other support from the American Association for Cancer Research, all outside of the submitted work. Mototsugu Oya has received consultancy or advisory fees from Bayer; honoraria from Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb Japan, Chugai, Janssen, MSD, Novartis, Ono Pharmaceuticals, Pfizer, Sanofi, and Takeda and research funding from Astellas Pharma, Novartis, and Pfizer. Mikio Sugimoto has received honoraria from Astellas, AstraZeneca, Janssen Pharmaceuticals, and Takeda; research funding from Astellas, AstraZeneca, Bristol Myers Squibb, Janssen, MSD, and Pfizer; and travel and accommodation expenses from Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Janssen, and MSD. Rana R. McKay has received institutional grant/research support from AstraZeneca, Bayer, Bristol Myers Squibb, Exelixis, Oncternal, and Tempus; consultancy fees from Arcus, Ambrx, AstraZeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera, Caris, Dendreon, Eisai, Exelixis, Johnson & Johnson, Lilly, Merck, Myovant, NeoMorph, Novartis, Pfizer, Sanofi, SeaGen, Sorrento Therapeutics, Telix, and Tempus. Maha Hussain has received honoraria from AstraZeneca, Bayer, Convergent, Novartis, and Tango; has served on advisory boards and received honoraria for the following invited educational events/lectures/manuscripts: PROST8 Consensus Conference Steering Committee member and session chair (honorarium received from MJH, RTP Lecture, AACR Satellite Symposium Lecture, Academic CME and travel expenses); South Africa Oncology Society lecture (honorarium received from AstraZeneca); Bayer APEX meeting (travel/accommodation, honorarium); 2nd International Genitourinary Cancer Conference Prostate Cancer Educational program (honorarium received from AstraZeneca); PER/NYGU – co-chair/speaker, RTP, Prostate Cancer Diagnostic Medical Education (Virtual) program (honorarium from AstraZeneca). Maha Hussain has also had clinical trials funding contracts with Arvinas, AstraZeneca, Bayer, and Northwestern University. Noel W. Clarke has received institutional consultancy or advisory fees from Astellas Pharma, Bayer, Ferring, Janssen-Cilag, and Sanofi; speaker bureau fees from Astellas Pharma, AstraZeneca, Bayer, Janssen-Cilag, Pfizer, and Sanofi; and travel and accommodation expenses associated with lectures and advisory board meetings from Astellas Pharma, AstraZeneca, Bayer, Ferring, Ipsen, Janssen-Cilag, and Sanofi. Ethics approval: Not applicable. Consent to participate: Not applicable. Consent for publication: Not applicable. Availability of data and material: No new data were generated or analyzed in support of this research. Code availability: Not applicable. Author contributions: All authors contributed to the concept and development of this review article, including drafting and critical revision of the manuscript. All authors have approved the submitted version.
Figures


References
-
- Kariburyo-Yay F, George DJ, Aggarwal H, Tepsick JG, Yu R, Li W, et al. Real-world baseline treatment patterns and overall survival (rwOS) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) treated with olaparib in the United States. J Clin Oncol. 2024;42(16 Suppl):5054. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

