Surface Charge Overrides Protein Corona Formation in Determining the Cytotoxicity, Cellular Uptake, and Biodistribution of Silver Nanoparticles
- PMID: 40397405
- PMCID: PMC12175132
- DOI: 10.1021/acsabm.5c00392
Surface Charge Overrides Protein Corona Formation in Determining the Cytotoxicity, Cellular Uptake, and Biodistribution of Silver Nanoparticles
Abstract
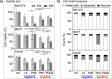
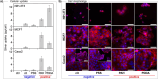
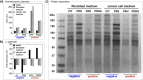
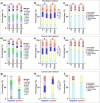
Silver nanoparticles (AgNPs) hold great promise in biomedical applications due to their unique properties and potential for specific tissue targeting. However, the clinical translation of nanoparticle-based therapeutics remains challenging, primarily due to an incomplete understanding of how nanoparticle properties influence interactions at the nano-bio interface, as well as the role of surface-adsorbed proteins (i.e., protein corona) in modulating nanoparticle-cell interactions. This study demonstrates that the surface charge has a greater influence than protein corona formation in determining the cytotoxicity, cellular uptake, and biodistribution of AgNPs. Using negatively and positively charged AgNPs, we show that while protein corona formation is essential for ensuring nanoparticle availability for cellular interactions, the adsorption of biomolecules is nonspecific and independent of surface charge. Conversely, the surface charge significantly influences the interactions of AgNPs with cells. Positively charged nanoparticles exhibit enhanced cellular uptake, preferential accumulation in lysosomes, and pronounced mitochondrial damage compared to their negatively charged counterparts, resulting in greater cytotoxic effects. This effect is particularly evident in human breast cancer cells, where negatively charged nanoparticles show minimal uptake and cytotoxicity. These findings demonstrate that surface charge is the primary factor governing nanoparticle-cell interactions rather than protein corona formation. Nonetheless, the protein corona plays a critical role in stabilizing nanoparticles in physiological environments.
Keywords: Biodistribution; Cell uptake; Cytotoxicity; Nanoparticles; Protein corona; Surface charge.
Figures


References
-
- Lee D., Huntoon K., Lux J., Kim B. Y. S., Jiang W.. Engineering nanomaterial physical characteristics for cancer immunotherapy. Nature Reviews Bioengineering. 2023;1(7):499–517. doi: 10.1038/s44222-023-00047-3. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
