CD83 suppresses endogenous March-I-dependent MHC class II ubiquitination, endocytosis, and degradation
- PMID: 40397676
- PMCID: PMC12130838
- DOI: 10.1073/pnas.2504077122
CD83 suppresses endogenous March-I-dependent MHC class II ubiquitination, endocytosis, and degradation
Abstract
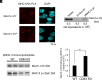
MHC class II glycoproteins (MHC-II) bind peptides derived from exogenous antigens and dendritic cells (DCs) present these peptide MHC-II (pMHC-II) complexes to antigen-specific CD4 T cells during immune responses. The turnover of surface pMHC-II on antigen-presenting cells (APCs) is controlled by ubiquitin-mediated degradation of pMHC-II by the E3 ubiquitin ligase March-I. To study March-I protein expression, we have generated a mouse in which a V5 epitope-tag was knocked-in to the endogenous March-I gene, thereby allowing us to follow the fate of March-I using high-affinity anti-V5 antibodies. Quantitative analysis revealed that resting spleen DCs and B cells express only ~500 and 125 March-I molecules/cell, respectively. Endogenous March-I protein has a very short half-life in DCs and March-I mRNA, March-I protein, and MHC-II ubiquitination are rapidly terminated upon activation of both DCs and B cells. Like March-I, CD83 is a known regulator of MHC-II expression in APCs and we also show that CD83 suppresses endogenous March-I-dependent MHC-II ubiquitination, endocytosis, and degradation in mouse spleen DCs. Thus, our study reveals molecular mechanisms for both March-I- and CD83-dependent regulation of MHC-II expression in APCs.
Keywords: MHC class II; antigen presentation; ubiquitination.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

