Personal care products disrupt the human oxidation field
- PMID: 40397733
- PMCID: PMC12094237
- DOI: 10.1126/sciadv.ads7908
Personal care products disrupt the human oxidation field
Abstract
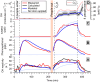
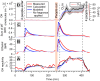
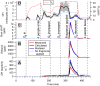
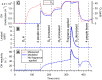
People generate hydroxyl radicals (OH) in the presence of ozone via the ozonolysis of skin-emitted alkenes. In this study, we found that the application of personal care products (PCPs) including fragrances and body lotions suppresses the human oxidation field. Body lotion hampers the generation of 6-methyl-5-hepten-2-one, a key OH precursor, while many volatile ingredients of PCPs enhance OH loss in the gas phase. Although fragrances contain terpenes capable of generating OH through ozonolysis, the much larger amount of ethanol solvent acts as a large OH sink. We combined a multiphase chemical kinetic model and a computational fluid dynamics model to demonstrate how the concentrations of the reactive components develop in the indoor environment. These findings have implications for the indoor chemistry of occupied spaces and human health.
Figures
References
-
- Gligorovski S., Abbatt J. P. D., An indoor chemical cocktail. Science 359, 632–633 (2018). - PubMed
-
- Klepeis N. E., Nelson W. C., Ott W. R., Robinson J. P., Tsang A. M., Switzer P., Behar J. V., Hern S. C., Engelmann W. H., The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 11, 231–252 (2001). - PubMed
-
- Nassikas N. J., McCormack M. C., Ewart G., Balmes J. R., Bond T. C., Brigham E., Cromar K., Goldstein A. H., Hicks A., Hopke P. K., Meyer B., Nazaroff W. W., Paulin L. M., Rice M. B., Thurston G. D., Turpin B. J., Vance M. E., Weschler C. J., Zhang J., Kipen H. M., Indoor air sources of outdoor air pollution: Health consequences, policy, and recommendations: An official American Thoracic Society workshop report. Ann. Am. Thorac. Soc. 21, 365–376 (2024). - PMC - PubMed
-
- Bekö G., Wargocki P., Wang N., Li M., Weschler C. J., Morrison G., Langer S., Ernle L., Licina D., Yang S., Zannoni N., Williams J., The Indoor Chemical Human Emissions and Reactivity (ICHEAR) project: Overview of experimental methodology and preliminary results. Indoor Air 30, 1213–1228 (2020). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

