Neuronal lipofuscinosis caused by Kufs disease/CLN4 DNAJC5 mutations but not by a CSPα/DNAJC5 deficiency
- PMID: 40397740
- PMCID: PMC12094229
- DOI: 10.1126/sciadv.ads3393
Neuronal lipofuscinosis caused by Kufs disease/CLN4 DNAJC5 mutations but not by a CSPα/DNAJC5 deficiency
Abstract
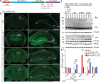
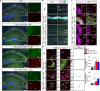
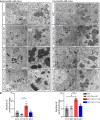
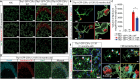
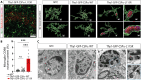
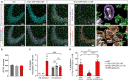
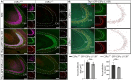
Kufs disease/CLN4 is an autosomal dominant neurodegenerative disorder caused by unknown mechanisms through Leu115Arg and Leu116Δ mutations in the DNAJC5 gene that encodes the synaptic vesicle co-chaperone cysteine string protein α (CSPα/DNAJC5). To investigate the disease mechanisms in vivo, we generated three independent mouse lines overexpressing different versions of CSPα/DNAJC5 under the neuron-specific Thy1 promoter: wild-type (WT), Leu115Arg, and Leu116Δ. Mice expressing mutant Leu115Arg CSPα/DNAJC5 are viable but develop motor deficits. As described in patients with Kufs disease, we observed the pathological lipofuscinosis and intracellular structures resembling granular osmiophilic deposits (GRODs) in the mutant but not in the WT transgenic lines. Microglia engulf lipofuscin and lipofuscin-containing neurons. Notably, conventional or conditional knockout mice lacking CSPα/DNAJC5 did not exhibit any signs of increased lipofuscinosis or GRODs. Our novel mouse models provide a valuable tool to investigate the molecular mechanisms underlying Kufs disease/CLN4. DNAJC5 mutations cause neuronal lipofuscinosis through a cell-autonomous gain of a pathological function of CSPα/DNAJC5.
Figures



References
-
- Butz E. S., Chandrachud U., Mole S. E., Cotman S. L., Moving towards a new era of genomics in the neuronal ceroid lipofuscinoses. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165571 (2020). - PubMed
-
- Nelvagal H. R., Lange J., Takahashi K., Tarczyluk-Wells M. A., Cooper J. D., Pathomechanisms in the neuronal ceroid lipofuscinoses. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165570 (2020). - PubMed
-
- Cárcel-Trullols J., Kovács A. D., Pearce D. A., Cell biology of the NCL proteins: What they do and don’t do. Biochim. Biophys. Acta Mol. Basis Dis. 1852, 2242–2255 (2015). - PubMed
-
- Boehme D. H., Cottrell J. C., Leonberg S. C., Zeman W., A dominant form of neuronal ceroid-lipofuscinosis. Brain 94, 745–760 (1971). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

