Genome-wide analyses of cell-free DNA for therapeutic monitoring of patients with pancreatic cancer
- PMID: 40397745
- PMCID: PMC12094228
- DOI: 10.1126/sciadv.ads5002
Genome-wide analyses of cell-free DNA for therapeutic monitoring of patients with pancreatic cancer
Abstract
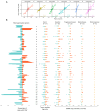
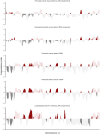
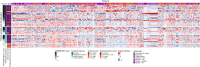
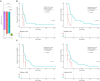
Determining response to therapy for patients with pancreatic cancer can be challenging. We evaluated methods for assessing therapeutic response using cell-free DNA (cfDNA) in plasma from patients with metastatic pancreatic cancer in the CheckPAC trial (NCT02866383). Patients were evaluated before and after initiation of therapy using tumor-informed plasma whole-genome sequencing (WGMAF) and tumor-independent genome-wide cfDNA fragmentation profiles and repeat landscapes (ARTEMIS-DELFI). Using WGMAF, molecular responders had a median overall survival (OS) of 319 days compared to 126 days for nonresponders [hazard ratio (HR) = 0.29, 95% confidence interval (CI) = 0.11-0.79, P = 0.011]. For ARTEMIS-DELFI, patients with low scores after therapy initiation had longer median OS than patients with high scores (233 versus 172 days, HR = 0.12, 95% CI = 0.046-0.31, P < 0.0001). We validated ARTEMIS-DELFI in patients with pancreatic cancer in the PACTO trial (NCT02767557). These analyses suggest that noninvasive mutation and fragmentation-based cfDNA approaches can identify therapeutic response of individuals with pancreatic cancer.
Figures




Similar articles
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
-
Detection of Brain Cancer Using Genome-wide Cell-free DNA Fragmentomes.Cancer Discov. 2025 Aug 4;15(8):1593-1608. doi: 10.1158/2159-8290.CD-25-0074. Cancer Discov. 2025. PMID: 40299319 Free PMC article.
-
Cell-Free DNA in Metastatic Colorectal Cancer: A Systematic Review and Meta-Analysis.Oncologist. 2017 Sep;22(9):1049-1055. doi: 10.1634/theoncologist.2016-0178. Epub 2017 Aug 4. Oncologist. 2017. PMID: 28778958 Free PMC article.
-
The vitamin D receptor gene as a determinant of survival in pancreatic cancer patients: Genomic analysis and experimental validation.PLoS One. 2018 Aug 14;13(8):e0202272. doi: 10.1371/journal.pone.0202272. eCollection 2018. PLoS One. 2018. PMID: 30107003 Free PMC article. Clinical Trial.
-
Chemotherapy and radiotherapy for advanced pancreatic cancer.Cochrane Database Syst Rev. 2024 Dec 5;12(12):CD011044. doi: 10.1002/14651858.CD011044.pub3. Cochrane Database Syst Rev. 2024. PMID: 39635901
References
-
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., Bray F., Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021). - PubMed
-
- Siegel R. L., Miller K. D., Wagle N. S., Jemal A., Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023). - PubMed
-
- Ducreux M., Cuhna A. S., Caramella C., Hollebecque A., Burtin P., Goéré D., Seufferlein T., Haustermans K., Laethem J. L. V., Conroy T., Arnold D., ESMO Guidelines Committee , Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 26, v56–v68 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

