Sphingolipid synthesis in tumor-associated macrophages confers immunotherapy resistance in hepatocellular carcinoma
- PMID: 40397754
- PMCID: PMC12094245
- DOI: 10.1126/sciadv.adv0558
Sphingolipid synthesis in tumor-associated macrophages confers immunotherapy resistance in hepatocellular carcinoma
Abstract
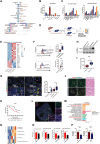
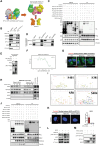
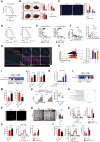
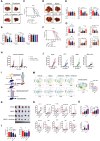
Dysregulated metabolism of immune cells in the tumor microenvironment leads to immune evasion and tumor progression. As a major cell component in the tumor, the metabolic reprogramming of tumor-associated macrophages (TAMs) creates an immunosuppressive microenvironment in hepatocellular carcinoma (HCC). Our study found that sphingolipid (particularly, sphingosine-1-phosphate or S1P) levels are a clinical indicator for prognosis and immunotherapy response in patients with HCC. S1P primarily derived from TAMs, where NIMA-related kinase 2 (NEK2) plays a key role in controlling the activity of serine palmitoyl-CoA transferase, a rate-limiting enzyme in S1P biosynthesis. The S1P produced by NEK2hi TAMs promotes hepatic tumor progression and confers immunotherapy resistance. Targeting S1P synthesis with a NEK2 inhibitor or S1P antagonist disrupted the immunosuppressive function of macrophages, shifted regulatory T cells (Tregs) to TH17 cells, and increased the number and activity of tumor-infiltrating T effectors, thereby enhancing antitumor efficacy in synergy with immune checkpoint blockade therapy.
Figures




References
-
- Bray F., Laversanne M., Sung H., Ferlay J., Siegel R. L., Soerjomataram I., Jemal A., Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024). - PubMed
-
- Vitale I., Manic G., Coussens L. M., Kroemer G., Galluzzi L., Macrophages and metabolism in the tumor microenvironment. Cell Metab. 30, 36–50 (2019). - PubMed
-
- Xiao J., Wang S., Chen L., Ding X., Dang Y., Han M., Zheng Y., Shen H., Wu S., Wang M., Yang D., Li N., Dong C., Hu M., Su C., Li W., Hui L., Ye Y., Tang H., Wei B., Wang H., 25-Hydroxycholesterol regulates lysosome AMP kinase activation and metabolic reprogramming to educate immunosuppressive macrophages. Immunity 57, 1087–1104.e7 (2024). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

