Core-Shell ZnO2@Cerium-Based Metal-Organic Framework with Low Turnover, Dual-Catalytic Activity for Biosafe Biofilm Dispersal and Immune Modulation
- PMID: 40397809
- PMCID: PMC12147083
- DOI: 10.1021/acsami.5c08974
Core-Shell ZnO2@Cerium-Based Metal-Organic Framework with Low Turnover, Dual-Catalytic Activity for Biosafe Biofilm Dispersal and Immune Modulation
Abstract
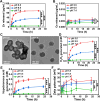
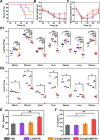
The era of relying on antibiotics for curing bacterial infections is rapidly approaching an end, necessitating development of non-antibiotic-based infection-control strategies. Dispersal of infectious biofilms is a potential strategy but yields dispersed bacteria in blood that may cause sepsis. We report a bromide-loaded, core-shell ZnO2-nanoparticle/Ce-based metal-organic framework (ZnO2@CeMOF/Br) of which the ZnO2 core degrades at pH ≤ 6.5, leaving the MOF's Ce node intact. ZnO2-core degradation initially generates a nonradical, relatively stable, low-oxidative hydrogen peroxide that can cleave matrix DNA causing dispersal of Staphylococcus aureus biofilms and reacts with bromide ions to form transient hypobromous acid. Hypobromous acid modulates macrophage polarization toward an M1-like phenotype to clear dispersed bacteria from blood. Subsequently the Ce3+/Ce4+ redox couple forming the Ce node acts as an electron shuttle upon oxidation/reduction to faciltate two catalytic reactions, maintaining hydrolysis of phosphodiester bonds and associated cleavage of matrix DNA as well as modulation of macrophage polarization. Neither growth of tissue cells or macrophages nor hemolysis are negatively affected by exposure to ZnO2@CeMOF/Br nanocatalysts at a ZnO2 nanoparticle over CeMOFs weight ratio ≤ 1.2, up until CeMOF concentrations less than at least 180 μg/mL. Under biosafe, low-turnover catalytic conditions, irrigation of infected wounds in diabetic mice with ZnO2@CeMOF/Br nanocatalysts (90 μg/mL) results in 100% survival, fast recovery of healthy body temperature and weight, lower numbers of CFUs in blood and wound and organ tissues, and macrophage polarization toward an M1-like phenotype, demonstrating potential of ZnO2@CeMOF/Br nanocatalysts for non-antibiotic-based infection control.
Keywords: biofilm; extracellular DNA; immune modulation; metal−organic framework; sepsis; turnover frequency; turnover number.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

