Noninvasive Assessment of the Risk Features of Hemorrhage in Moyamoya Disease Using 7T MRI
- PMID: 40397837
- PMCID: PMC12096881
- DOI: 10.1212/WNL.0000000000213617
Noninvasive Assessment of the Risk Features of Hemorrhage in Moyamoya Disease Using 7T MRI
Abstract
Background and objectives: While digital subtraction angiography (DSA) is traditionally used for moyamoya disease (MMD) assessment, its invasiveness and limitations necessitate alternative methods. The higher signal-to-noise ratio (SNR) and contrast-to-noise ratio of 7T MRI improve the clarity of the image and retains the details of the structures. We aimed to assess the performance of 7T MRI in identifying hemorrhagic risk features of MMD compared with 3T MRI and DSA.
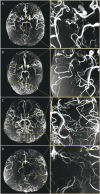
Methods: This cross-sectional study recruited patients with MMD who underwent both 7T and 3T MRI scans within a 24-hour window, from March 2022 to December 2023. Patients were categorized into hemorrhagic, ischemic, and asymptomatic groups based on standard MRI findings and clinical symptoms. Corresponding DSA images acquired within 90 days were also collected as a comparative benchmark. Hemorrhage risk factors including dilatation and branch extension of the anterior choroidal artery (AChA) and posterior communicating artery (PComA) were assessed and graded on time-of-flight magnetic resonance angiography (TOF-MRA) and DSA images following established protocols. The hemorrhage locations were classified into anterior and posterior circulation groups.
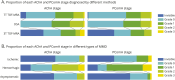
Results: A total of 180 patients (mean age, 43.95 ± 11.02 [SD] years; 53.9% female) were included in the study (hemorrhagic = 51, ischemic = 37, asymptomatic = 92). Notably, 42.4% of AChA and 27.7% of PComA anomalies detected on 7T TOF-MRA were absent on 3T imaging. The 7T TOF-MRA demonstrated a strong correlation with DSA in assessing the AChA stage (weighted κ = 0.891, p < 0.001) and PComA stage (weighted κ = 0.761, p < 0.001). Higher AChA (70.6% vs 21.6% vs 6.5%, p < 0.001) and PComA (51.0% vs 8.1% vs 12.0%, p < 0.001) grades were more common in patients with hemorrhagic MMD compared with ischemic and asymptomatic groups. In binary logistic regression analysis for hemorrhagic and ischemic groups, elevated stages of AChA (odds ratio [OR] 1.90, 95% CI 1.20-3.54, p = 0.042) and PComA (OR 3.89, 95% CI 1.76-8.58, p = 0.001) were associated with increased hemorrhagic risk. Furthermore, the proportion of higher AChA (62.2%, p = 0.008) and PComA (51.3%, p = 0.010) grades were more prevalent in cases involving both anterior and posterior circulations.
Discussion: The 7T TOF-MRA visualization of dilatation and branching extension of the AChA and PComA indicates a heightened risk of hemorrhage, suggesting that this imaging technique could serve as a valuable noninvasive tool for identifying hemorrhagic vulnerabilities in MMD.
Trial registration information: ClinicalTrials.gov, NCT05287750, Brain Diseases on 7.0T Magnetic Resonance Imaging, First Submitted January 2022. clinicaltrials.gov/study/NCT05287750.
Classification of evidence: This study provides Class II evidence that 7T-TOF MRA accurately distinguishes hemorrhagic risk in patients with MMD compared with 3T-TOF MRA and DSA.
Conflict of interest statement
The authors report no relevant disclosures. Go to
Figures



Similar articles
-
Hemorrhagic Moyamoya Angiopathy in European Patients.Stroke. 2024 Nov;55(11):2661-2668. doi: 10.1161/STROKEAHA.124.046859. Epub 2024 Sep 18. Stroke. 2024. PMID: 39291379
-
Duplex ultrasound for diagnosing symptomatic carotid stenosis in the extracranial segments.Cochrane Database Syst Rev. 2022 Jul 11;7(7):CD013172. doi: 10.1002/14651858.CD013172.pub2. Cochrane Database Syst Rev. 2022. PMID: 35815652 Free PMC article.
-
Age-Dependent Risk Factors for Hemorrhagic Events in Moyamoya Disease: The Role of Collateral Vessels.World Neurosurg. 2025 Jun;198:124000. doi: 10.1016/j.wneu.2025.124000. Epub 2025 Apr 21. World Neurosurg. 2025. PMID: 40268187
-
Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD011551. doi: 10.1002/14651858.CD011551.pub2. Cochrane Database Syst Rev. 2018. PMID: 29357120 Free PMC article.
-
Brain Morphometrics Correlations With Age Among 350 Participants Imaged With Both 3T and 7T MRI: 7T Improves Statistical Power and Reduces Required Sample Size.Hum Brain Mapp. 2025 Mar;46(4):e70195. doi: 10.1002/hbm.70195. Hum Brain Mapp. 2025. PMID: 40083197 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
